1. Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer--part 1: epidemiology, presentation, and prevention. BMJ. 2010; 341:c4684. PMID:
20855405.

2. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45(4-5):309–316. PMID:
18804401.


3. Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988; 48(11):3282–3287. PMID:
3365707.

4. Rusin P, Markiewicz L, Majsterek I. Genetic predeterminations of head and neck cancer. Postepy Hig Med Dosw. 2008; 62:490–501.
5. Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009; 17(11):1345–1351. PMID:
19618218.


6. Buglione M, Cavagnini R, Di Rosario F, Sottocornola L, Maddalo M, Vassalli L, et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Dental pathologies and osteoradionecrosis (Part 1) literature review and consensus statement. Crit Rev Oncol Hematol. 2016; 97:131–142. PMID:
26318095.


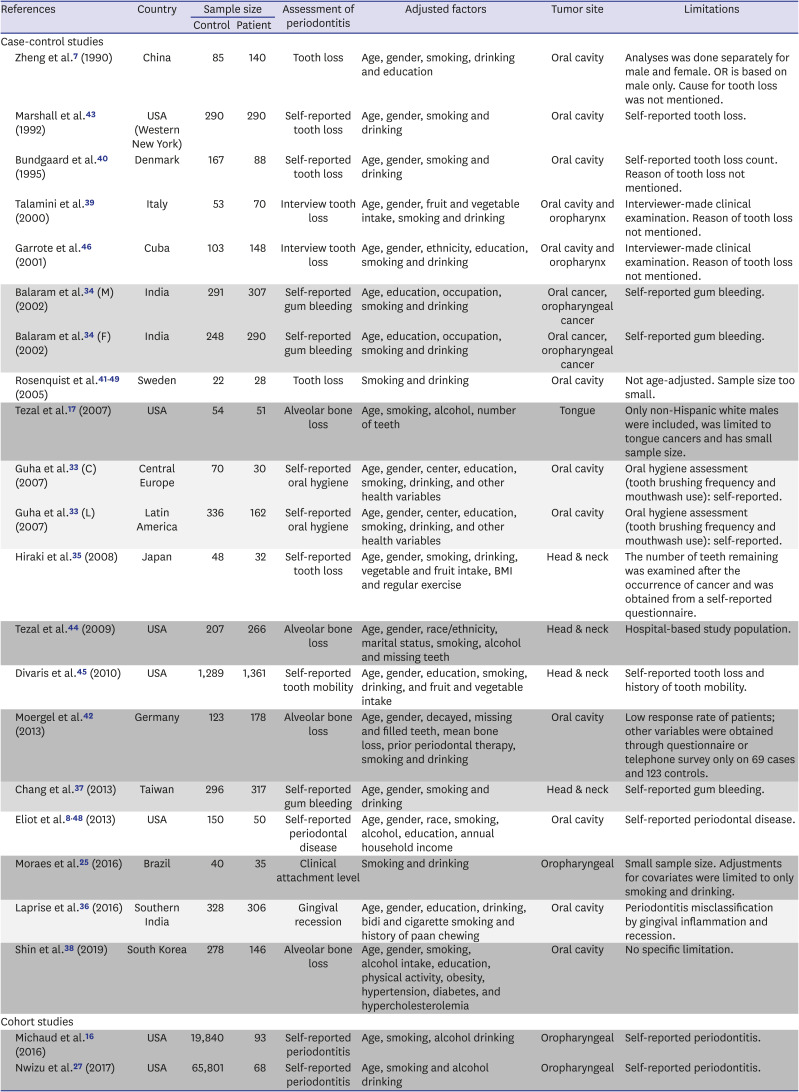
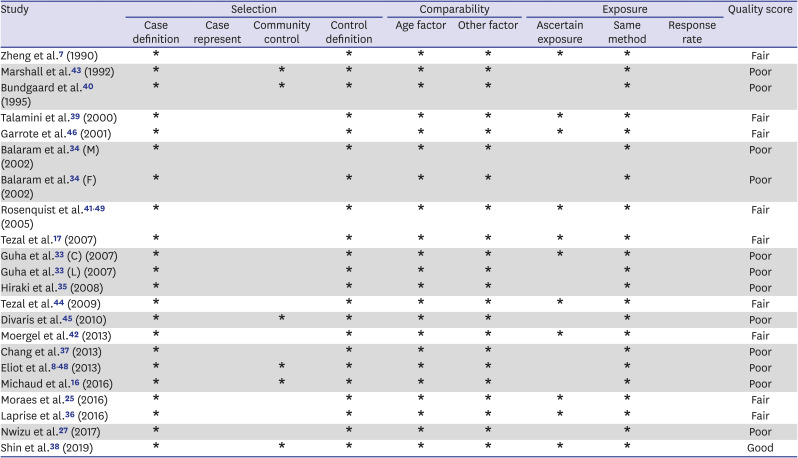
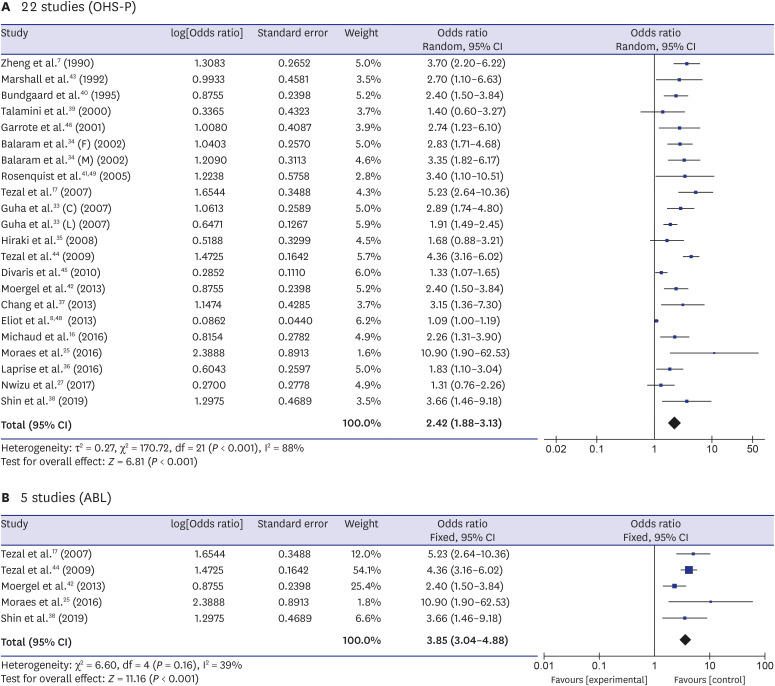
7. Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, et al. Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People's Republic of China. Cancer Causes Control. 1990; 1(3):235–241. PMID:
2102296.


8. Eliot MN, Michaud DS, Langevin SM, McClean MD, Kelsey KT. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Causes Control. 2013; 24(7):1315–1322. PMID:
23568534.



9. Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit Rev Oncol Hematol. 2016; 97:197–205. PMID:
26343577.


10. Conway DI, Hashibe M, Boffetta P, Wunsch-Filho V, Muscat J, La Vecchia C, et al. Enhancing epidemiologic research on head and neck cancer: INHANCE - The international head and neck cancer epidemiology consortium. Oral Oncol. 2009; 45(9):743–746. PMID:
19442571.


11. Saygun I, Kubar A, Ozdemir A, Slots J. Periodontitis lesions are a source of salivary cytomegalovirus and Epstein-Barr virus. J Periodontal Res. 2005; 40(2):187–191. PMID:
15733155.


12. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008; 83(4):489–501. PMID:
18380996.


13. Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006; 124(4):823–835. PMID:
16497591.


14. Ji S, Choi YS, Choi Y. Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodontal Res. 2015; 50(5):570–585. PMID:
25487426.


15. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357(9255):539–545. PMID:
11229684.


16. Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 2016; 27(5):941–947. PMID:
26811350.



17. Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007; 133(5):450–454. PMID:
17515503.


18. Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol. 2005; 76(3):406–410. PMID:
15857075.


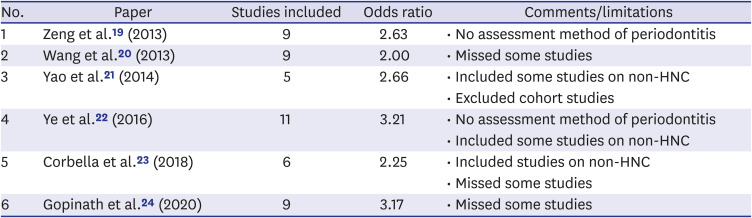
19. Zeng XT, Luo W, Huang W, Wang Q, Guo Y, Leng WD. Tooth loss and head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013; 8(11):e79074. PMID:
24260154.

20. Wang RS, Hu XY, Gu WJ, Hu Z, Wei B. Tooth loss and risk of head and neck cancer: a meta-analysis. PLoS One. 2013; 8(8):e71122. PMID:
23990929.

21. Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: a meta-analysis. Tumour Biol. 2014; 35(7):7073–7077. PMID:
24756759.


22. Ye L, Jiang Y, Liu W, Tao H. Correlation between periodontal disease and oral cancer risk: a meta-analysis. J Cancer Res Ther. 2016; 12(Suppl):C237–C240. PMID:
28230025.

23. Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One. 2018; 13(4):e0195683. PMID:
29664916.

24. Gopinath D, Kunnath Menon R, K Veettil S, George Botelho M, Johnson NW. Periodontal diseases as putative risk factors for head and neck cancer: systematic review and meta-analysis. Cancers (Basel). 2020; 12(7):E1893. PMID:
32674369.

25. Moraes RC, Dias FL, Figueredo CM, Fischer RG. Association between chronic periodontitis and oral/oropharyngeal cancer. Braz Dent J. 2016; 27(3):261–266. PMID:
27224557.


26. Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008; 9(6):550–558. PMID:
18462995.



27. Nwizu NN, Marshall JR, Moysich K, Genco RJ, Hovey KM, Mai X, et al. Periodontal disease and incident cancer risk among postmenopausal women: results from the women's health initiative observational cohort. Cancer Epidemiol Biomarkers Prev. 2017; 26(8):1255–1265. PMID:
28765338.



28. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998; 280(19):1690–1691. PMID:
9832001.

29. Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004; 160(4):301–305. PMID:
15286014.


31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11):1539–1558. PMID:
12111919.


32. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557–560. PMID:
12958120.



33. Guha N, Boffetta P, Wünsch Filho V, Eluf Neto J, Shangina O, Zaridze D, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007; 166(10):1159–1173. PMID:
17761691.


34. Balaram P, Sridhar H, Rajkumar T, Vaccarella S, Herrero R, Nandakumar A, et al. Oral cancer in southern India: the influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002; 98(3):440–445. PMID:
11920597.


35. Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008; 17(5):1222–1227. PMID:
18483345.


36. Laprise C, Shahul HP, Madathil SA, Thekkepurakkal AS, Castonguay G, Varghese I, et al. Periodontal diseases and risk of oral cancer in Southern India: results from the HeNCe Life study. Int J Cancer. 2016; 139(7):1512–1519. PMID:
27215979.


37. Chang JS, Lo HI, Wong TY, Huang CC, Lee WT, Tsai ST, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013; 49(10):1010–1017. PMID:
23948049.


38. Shin YJ, Choung HW, Lee JH, Rhyu IC, Kim HD. Association of Periodontitis with oral cancer: a case-control study. J Dent Res. 2019; 98(5):526–533. PMID:
30779879.


39. Talamini R, Vaccarella S, Barbone F, Tavani A, La Vecchia C, Herrero R, et al. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer. 2000; 83(9):1238–1242. PMID:
11027440.



40. Bundgaard T, Wildt J, Frydenberg M, Elbrønd O, Nielsen JE. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995; 6(1):57–67. PMID:
7718736.


41. Rosenquist K. Risk factors in oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Swed Dent J Suppl. 2005; (179):1–66.
42. Moergel M, Kämmerer P, Kasaj A, Armouti E, Alshihri A, Weyer V, et al. Chronic periodontitis and its possible association with oral squamous cell carcinoma - a retrospective case control study. Head Face Med. 2013; 9(1):39. PMID:
24321243.



43. Marshall JR, Graham S, Haughey BP, Shedd D, O'Shea R, Brasure J, et al. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992; 28B(1):9–15. PMID:
1422474.


44. Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009; 18(9):2406–2412. PMID:
19745222.


45. Divaris K, Olshan AF, Smith J, Bell ME, Weissler MC, Funkhouser WK, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010; 21(4):567–575. PMID:
20049634.



46. Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L, et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001; 85(1):46–54. PMID:
11437401.

47. Sultana J, Bashar A, Molla MR. New management strategies of oral tongue cancer in Bangladesh. J Maxillofac Oral Surg. 2014; 13(4):394–400. PMID:
26225002.


48. Eliot MN, Michaud DS, Langevin SM, McClean MD, Kelsey KT. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Causes Control. 2013; 24(7):1315–1322. PMID:
23568534.



49. Rosenquist K, Wennerberg J, Schildt EB, Bladström A, Göran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005; 125(12):1327–1336. PMID:
16303683.


50. Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005; 3(1):27. PMID:
15987522.


51. Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol. 2005; 21(1):32–38. PMID:
15687882.

52. Zarkin BA, Lillemoe KD, Cameron JL, Effron PN, Magnuson TH, Pitt HA. The triad of Streptococcus bovis bacteremia, colonic pathology, and liver disease. Ann Surg. 1990; 211(6):786–791. PMID:
2357141.



53. Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, et al. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005; 11(3):151–156. PMID:
15888105.


54. Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004; 34(1):9–21. PMID:
14717852.


55. Papapanou PN, Wennström JL. Radiographic and clinical assessments of destructive periodontal disease. J Clin Periodontol. 1989; 16(9):609–612. PMID:
2794096.

56. Rezende CP, Ramos MB, Daguíla CH, Dedivitis RA, Rapoport A. Oral health changes in with oral and oropharyngeal cancer. Rev Bras Otorrinolaringol (Engl Ed). 2008; 74(4):596–600.
57. Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011; 90(6):752–758. PMID:
21406610.



58. Wei J, Xie G, Zhou Z, Shi P, Qiu Y, Zheng X, et al. Salivary metabolite signatures of oral cancer and leukoplakia. Int J Cancer. 2011; 129(9):2207–2217. PMID:
21190195.


59. Shankarram V, Narayanan ML, Sudhakar MU, et al. Detection of oxidative stress in periodontal disease and oral cancer. Biomed Pharmacol J. 2015; 8(2):725–729.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download