This article has been
cited by other articles in ScienceCentral.
Abstract
Hospital-based surveillance for adverse events was conducted on healthcare workers after they received the first dose of coronavirus disease 2019 (COVID-19) vaccine. Among the two new platform vaccines (messenger RNA- and adenoviral vector-based vaccines), the rates of systemic adverse events were significantly higher among adenovirus-vectored vaccine recipients. Fatigue (87.6% vs. 53.8%), myalgia (80.8% vs. 50.0%), headache (72.0% vs. 28.8%), and fever (≥ 38.0°C, 38.7% vs. 0%) were the most common adverse events among adenovirus-vectored vaccine recipients, but most symptoms resolved within 2 days. Both types of COVID-19 vaccines were generally safe, and serious adverse events rarely occurred.
Keywords: COVID-19, Vaccine, Safety, Adverse Event, Surveillance
The coronavirus disease 2019 (COVID-19) pandemic has spread worldwide, and each country is competing for COVID-19 vaccines. Vaccination has rapidly progressed to protect high-risk groups and obtain herd immunity in the general population. New messenger RNA (mRNA)- and viral vector-based COVID-19 vaccines have been produced. Although they were approved based on data from large-scale phase 3 clinical trials, there have been concerns about unexpected adverse events after vaccination.
12 Since February 26, 2021, in South Korea, two COVID-19 vaccines (Pfizer-BioNTech and AstraZeneca) have been administered in two doses. As of March 23, 2021, 680,560 received the first dose.
3
In South Korea, healthcare workers received the highest priority for COVID-19 vaccination, followed by elderly people in long-term care facilities. This study aimed to identify adverse events occurring 7 days after the first dose of COVID-19 vaccination in 6,218 vaccinated healthcare workers of three Korea University hospitals. A total of 5,930 healthcare workers received AstraZeneca vaccine (chimpanzee adenovirus-vectored vaccine, 0.5 mL [5 × 10
10 viral particles] per dose), and 288 received Pfizer-BioNTech vaccine (mRNA vaccine; 0.3 mL [30 μg] per dose). We conducted a Google survey on adverse events following COVID-19 vaccination in healthcare workers (
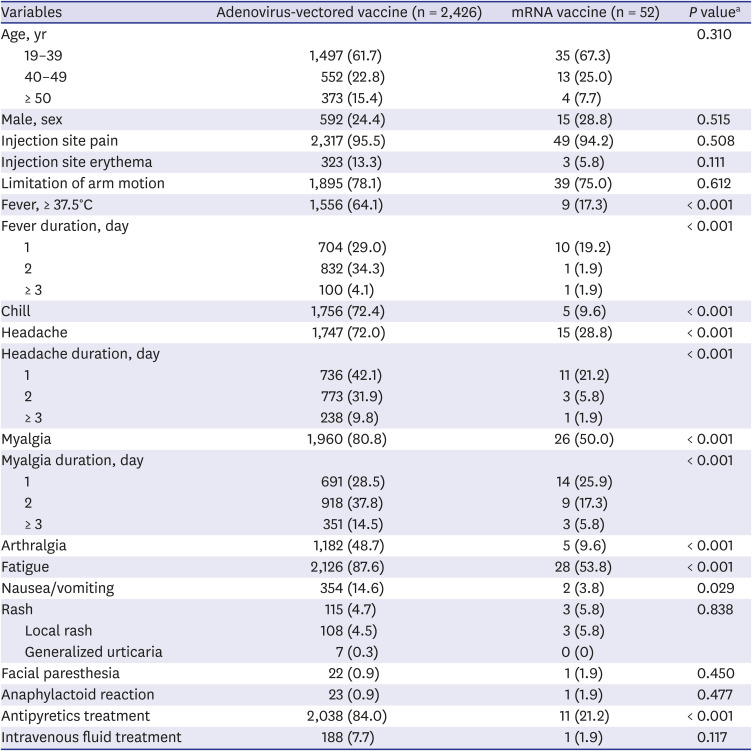
Table 1). Among 6,218 COVID-19 vaccine recipients, 2,478 (39.9%) responded to the survey. Consistent with data from clinical trials,
4 fatigue (87.6%), myalgia (80.8%), headache (72.0%), and fever (≥ 37.5°C, 64.1%; ≥ 38.0°C, 38.7%) were the most common adverse events among adenovirus-vectored vaccine recipients (
Table 1 and
Supplementary Table 1). In the phase 2/3 ChadOx1 nCoV-19 vaccine clinical trial, 42.9% of participants aged 18–55 years complained of fever, and 24.5% had documented fever (≥ 38.0°C) after standard dose priming.
4 In this survey, compared to the clinical trial,
4 a higher proportion of adenovirus-vectored vaccine recipients reported fever, but 98.7% (1,536/1,556) resolved within 2 days after vaccination. When comparing adverse events among the participants that received the two types of COVID-19 vaccines, there was no significant difference in local reactions. The overall rates of systemic adverse events (fever, chills, headache, myalgia, arthralgia, fatigue, and nausea/vomiting) were significantly higher in adenovirus-vectored vaccine recipients than in mRNA vaccine recipients. More than 80% of adenovirus-vectored vaccine recipients received antipyretic agents, while approximately 20% of mRNA vaccine recipients received antipyretic medications. After the first dose of mRNA vaccination, the rate of systemic adverse events was significantly lower than that of the adenovirus-vectored vaccine. However, the incidence rate of mRNA vaccine-related systemic adverse events in this survey was higher than that of the clinical trial data: fever (≥ 38.0°C, 4%), muscle pain (21%), headache (39%), and fatigue (47%) in participants aged 16–55 years.
2 For the adenovirus-vectored vaccine, the rate of adverse events after the first dose was high, and the rate of adverse events after the second dose was relatively low in the phase 2/3 clinical trial.
4 For the mRNA vaccines, the rate of adverse events after the second dose was higher than that of the first dose vaccination in a phase 3 clinical trial.
2 Thus, it was difficult to compare the safety of the different types of vaccines using only the results from the first-dose vaccination.
Table 1
Adverse events reported within 7 days after the first dose coronavirus disease 2019 vaccination
|
Variables |
Adenovirus-vectored vaccine (n = 2,426) |
mRNA vaccine (n = 52) |
P valuea
|
|
Age, yr |
|
|
0.310 |
|
19–39 |
1,497 (61.7) |
35 (67.3) |
|
40–49 |
552 (22.8) |
13 (25.0) |
|
≥ 50 |
373 (15.4) |
4 (7.7) |
|
Male, sex |
592 (24.4) |
15 (28.8) |
0.515 |
|
Injection site pain |
2,317 (95.5) |
49 (94.2) |
0.508 |
|
Injection site erythema |
323 (13.3) |
3 (5.8) |
0.111 |
|
Limitation of arm motion |
1,895 (78.1) |
39 (75.0) |
0.612 |
|
Fever, ≥ 37.5°C |
1,556 (64.1) |
9 (17.3) |
< 0.001 |
|
Fever duration, day |
|
|
< 0.001 |
|
1 |
704 (29.0) |
10 (19.2) |
|
2 |
832 (34.3) |
1 (1.9) |
|
≥ 3 |
100 (4.1) |
1 (1.9) |
|
Chill |
1,756 (72.4) |
5 (9.6) |
< 0.001 |
|
Headache |
1,747 (72.0) |
15 (28.8) |
< 0.001 |
|
Headache duration, day |
|
|
< 0.001 |
|
1 |
736 (42.1) |
11 (21.2) |
|
2 |
773 (31.9) |
3 (5.8) |
|
≥ 3 |
238 (9.8) |
1 (1.9) |
|
Myalgia |
1,960 (80.8) |
26 (50.0) |
< 0.001 |
|
Myalgia duration, day |
|
|
< 0.001 |
|
1 |
691 (28.5) |
14 (25.9) |
|
2 |
918 (37.8) |
9 (17.3) |
|
≥ 3 |
351 (14.5) |
3 (5.8) |
|
Arthralgia |
1,182 (48.7) |
5 (9.6) |
< 0.001 |
|
Fatigue |
2,126 (87.6) |
28 (53.8) |
< 0.001 |
|
Nausea/vomiting |
354 (14.6) |
2 (3.8) |
0.029 |
|
Rash |
115 (4.7) |
3 (5.8) |
0.838 |
|
Local rash |
108 (4.5) |
3 (5.8) |
|
Generalized urticaria |
7 (0.3) |
0 (0) |
|
Facial paresthesia |
22 (0.9) |
1 (1.9) |
0.450 |
|
Anaphylactoid reaction |
23 (0.9) |
1 (1.9) |
0.477 |
|
Antipyretics treatment |
2,038 (84.0) |
11 (21.2) |
< 0.001 |
|
Intravenous fluid treatment |
188 (7.7) |
1 (1.9) |
0.117 |

Along with the Google survey, passive surveillance for adverse events of special interest (AESI) was conducted. Vaccine recipients with adverse events that were not controlled by supportive treatment were referred to the emergency room or infectious disease department. They were additionally linked to other departments when necessary. Patients, who visited the emergency room or clinics, reported the adverse events to the infection control unit, and the infectious disease specialist evaluated the causality of vaccination and adverse events. According to the passive surveillance for AESI included in the priority list by the Brighton Collaboration,
5 a case of facial palsy developed after the administration of an adenovirus-vectored vaccine. In addition, severe adverse events were reported among adenovirus-vectored vaccine recipients. These included anaphylactoid reaction (one case), angioedema (one case), generalized urticaria (two cases), cellulitis (four cases), and paresthesia of the unilateral face and injected arm (two cases). There was a discrepancy between the active and passive surveillance results for severe adverse events after the COVID-19 vaccination. More than 20 vaccine recipients reported anaphylactoid reactions and facial paresthesia on Google survey, but most of them were clinically insignificant (
Table 1).
In the UK, 10.9 million first doses of the Pfizer-BioNTech vaccine and 13.7 million doses of the AstraZeneca vaccine had been administered, and around 1.3 million second doses, mostly the Pfizer-BioNTech vaccine, had been administered up to 14 March 2021.
6 In the UK, the safety of all healthcare products has been monitored by the Medicines and Healthcare products Regulatory Agency using Yellow Card Scheme. According to the weekly summary of Yellow Card reporting after COVID-19 vaccination in the UK, the majority reports were injection-site reactions and generalized symptoms such as flu-like illness, headache, chills, fatigue, nausea, fever, dizziness, and muscle ache.
6 Similar to the results of this study, most adverse reactions resolved within a few days of vaccination.
6
This study had some limitations. First, the sample size was small, so we could not identify AESI enough through this surveillance for healthcare workers. It will be necessary to operate a reinforced monitoring system for AESI, including immune thrombocytopenia, disseminated intravascular coagulation, and cerebral venous sinus thrombosis. Second, the Google survey was not conducted on all vaccine recipients, so the incidence rate of adverse events might have been overestimated due to the high response rate among those with adverse events.
In summary, both types of COVID-19 vaccines were generally safe, and serious adverse events rarely occurred. However, the adenovirus-vectored vaccine recipients reported fever, headache, and muscle pain more frequently than expected. In addition, about 40% of the vaccine recipients complained of ≥ grade 2 adverse events that interfered with daily activities (
Supplementary Table 1). Nevertheless, most symptoms resolved within 2 days. A 1–2-day leave after vaccination may be considered. In the situation where mass immunization with new platform COVID-19 vaccines is in progress, a multi-center hospital-based monitoring system is vital in promptly evaluating and collecting data on serious adverse events.