1. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins-Obstetrics. Practice bulletin no. 172: premature rupture of membranes. Obstet Gynecol. 2016; 128(4):e165–e177. PMID:
27661655.
2. Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol. 2009; 201(3):230–240. PMID:
19733274.


3. Yeast JD. Preterm premature rupture of the membranes before viability. Clin Perinatol. 2001; 28(4):849–860. PMID:
11758532.


4. Xiao ZH, André P, Lacaze-Masmonteil T, Audibert F, Zupan V, Dehan M. Outcome of premature infants delivered after prolonged premature rupture of membranes before 25 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2000; 90(1):67–71. PMID:
10767513.


5. Hadi HA, Hodson CA, Strickland D. Premature rupture of the membranes between 20 and 25 weeks' gestation: role of amniotic fluid volume in perinatal outcome. Am J Obstet Gynecol. 1994; 170(4):1139–1144. PMID:
8166198.


6. Brumbaugh JE, Colaizy TT, Nuangchamnong N, O'Brien EA, Fleener DK, Rijhsinghani A, et al. Neonatal survival after prolonged preterm premature rupture of membranes before 24 weeks of gestation. Obstet Gynecol. 2014; 124(5):992–998. PMID:
25437729.


7. Kibel M, Asztalos E, Barrett J, Dunn MS, Tward C, Pittini A, et al. Outcomes of pregnancies complicated by preterm premature rupture of membranes between 20 and 24 weeks of gestation. Obstet Gynecol. 2016; 128(2):313–320. PMID:
27400016.


8. Muris C, Girard B, Creveuil C, Durin L, Herlicoviez M, Dreyfus M. Management of premature rupture of membranes before 25 weeks. Eur J Obstet Gynecol Reprod Biol. 2007; 131(2):163–168. PMID:
16846673.


9. Everest NJ, Jacobs SE, Davis PG, Begg L, Rogerson S. Outcomes following prolonged preterm premature rupture of the membranes. Arch Dis Child Fetal Neonatal Ed. 2008; 93(3):F207–11. PMID:
17660215.

10. Manuck TA, Eller AG, Esplin MS, Stoddard GJ, Varner MW, Silver RM. Outcomes of expectantly managed preterm premature rupture of membranes occurring before 24 weeks of gestation. Obstet Gynecol. 2009; 114(1):29–37. PMID:
19546755.


11. Melamed N, Hadar E, Ben-Haroush A, Kaplan B, Yogev Y. Factors affecting the duration of the latency period in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009; 22(11):1051–1056. PMID:
19900043.


12. Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2010; (8):CD001058. PMID:
20687063.

13. Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982; 59(5):539–545. PMID:
7070724.

14. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986; 155(3):471–479. PMID:
3752169.


15. Kilbride HW, Thibeault DW. Neonatal complications of preterm premature rupture of membranes. Pathophysiology and management. Clin Perinatol. 2001; 28(4):761–785. PMID:
11817188.


16. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996; 175(3 Pt 1):675–681. PMID:
8828433.


17. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990; 162(1):46–52. PMID:
2301516.


18. Winn HN, Chen M, Amon E, Leet TL, Shumway JB, Mostello D. Neonatal pulmonary hypoplasia and perinatal mortality in patients with midtrimester rupture of amniotic membranes--a critical analysis. Am J Obstet Gynecol. 2000; 182(6):1638–1644. PMID:
10871491.

19. Park GY, Park WS, Yoo HS, Ahn SY, Sung SI, Kim SS, et al. Short-term outcomes comparison between preterm infants with and without acute hypoxic respiratory failure attributable to presumed pulmonary hypoplasia after prolonged preterm premature rupture of membranes before 25 gestational weeks. J Matern Fetal Neonatal Med. 2019; 32(12):1938–1945. PMID:
29279020.


20. Park GY, Park WS, Sung SI, Kim MS, Lee MH, Jeon GW, et al. Neonatal outcome comparisons between preterm infants with or without early pulmonary hypertension following prolonged preterm premature rupture of membranes before 25 gestational weeks in Korean Neonatal Network. J Matern Fetal Neonatal Med. 2020; 1(6):1–9.

21. Phelan JP, Ahn MO, Smith CV, Rutherford SE, Anderson E. Amniotic fluid index measurements during pregnancy. J Reprod Med. 1987; 32(8):601–604. PMID:
3309290.

22. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163(7):1723–1729. PMID:
11401896.


23. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92(4):529–534. PMID:
305471.


24. Sarkar S, Shankaran S, Laptook AR, Sood BG, Do B, Stoll BJ, et al. Screening cranial imaging at multiple time points improves cystic periventricular leukomalacia detection. Am J Perinatol. 2015; 32(10):973–979. PMID:
25730135.


25. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978; 187(1):1–7. PMID:
413500.


26. Lee SM, Chang M, Kim KS. Blood culture proven early onset sepsis and late onset sepsis in very-low-birth-weight infants in Korea. J Korean Med Sci. 2015; 30(Suppl 1):S67–74. PMID:
26566360.

27. Kim JK, Chang YS, Sung S, Park WS. Mortality rate-dependent variations in the survival without major morbidities rate of extremely preterm infants. Sci Rep. 2019; 9(1):7371. PMID:
31089251.



28. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005; 123(7):991–999. PMID:
16009843.

29. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The model for understanding success in quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012; 21(1):13–20.


30. Gould JB. Quality improvement in perinatal medicine: assessing the quality of perinatal care. Neoreviews. 2004; 5(2):e33–41.

31. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996; 20(5):389–400. PMID:
8912993.


32. Manuck TA, Maclean CC, Silver RM, Varner MW. Preterm premature rupture of membranes: does the duration of latency influence perinatal outcomes? Am J Obstet Gynecol. 2009; 201(4):414.e1–414.e6. PMID:
19788972.

33. Shim JW, Jin HS, Bae CW. Changes in survival rate for very-low-birth-weight infants in Korea: comparison with other countries. J Korean Med Sci. 2015; 30(Suppl 1):S25–S34. PMID:
26566354.

34. Williams O, Hutchings G, Debieve F, Debauche C. Contemporary neonatal outcome following rupture of membranes prior to 25 weeks with prolonged oligohydramnios. Early Hum Dev. 2009; 85(5):273–277. PMID:
19108959.

35. Kurdoglu M, Kolusari A, Adali E, Yildizhan R, Kurdoglu Z, Kucukaydin Z, et al. Does residual amniotic fluid after preterm premature rupture of membranes have an effect on perinatal outcomes? 12 years experience of a tertiary care center. Arch Gynecol Obstet. 2010; 281(4):601–607. PMID:
19521709.


36. Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy. 2013; 2013:412831. PMID:
23533760.

37. Pugni L, Pietrasanta C, Acaia B, Merlo D, Ronchi A, Ossola MW, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016; 29(9):1525–1529. PMID:
26135227.


38. Vergani P, Ghidini A, Locatelli A, Cavallone M, Ciarla I, Cappellini A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994; 170(5 Pt 1):1359–1364. PMID:
8178866.


39. Pryles CV, Steg NL, Nair S, Gellis SS, Tenney B. A controlled study of the influence on the newborn of prolonged premature rupture of the amniotic membranes and/or infection in the mother. Pediatrics. 1963; 31:608–622. PMID:
13972386.

40. Drassinower D, Friedman AM, Običan SG, Levin H, Gyamfi-Bannerman C. Prolonged latency of preterm premature rupture of membranes and risk of neonatal sepsis. Am J Obstet Gynecol. 2016; 214(6):743.e1–743.e6. PMID:
26723194.

41. Melamed N, Ben-Haroush A, Pardo J, Chen R, Hadar E, Hod M, et al. Expectant management of preterm premature rupture of membranes: is it all about gestational age? Am J Obstet Gynecol. 2011; 204(1):48.e1–48.e8. PMID:
21074135.

42. McElrath TF, Allred EN, Leviton A. Development Epidemiology Network Investigators. Prolonged latency after preterm premature rupture of membranes: an evaluation of histologic condition and intracranial ultrasonic abnormality in the neonate born at <28 weeks of gestation. Am J Obstet Gynecol. 2003; 189(3):794–798. PMID:
14526316.

43. Gyamfi-Bannerman C, Son M. Preterm premature rupture of membranes and the rate of neonatal sepsis after two courses of antenatal corticosteroids. Obstet Gynecol. 2014; 124(5):999–1003. PMID:
25437730.


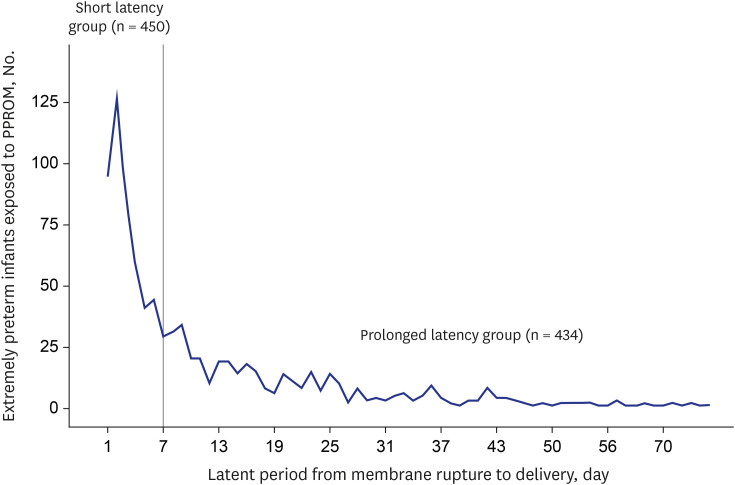
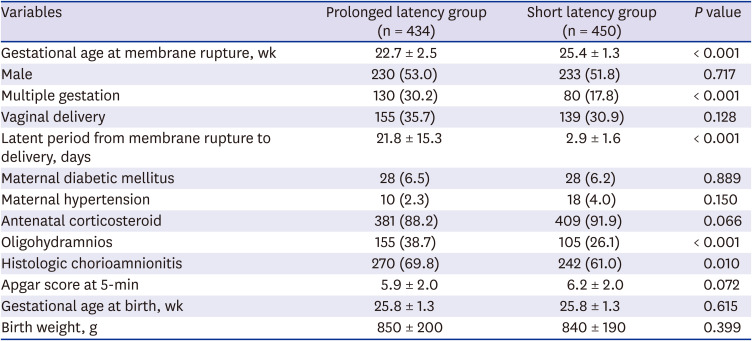
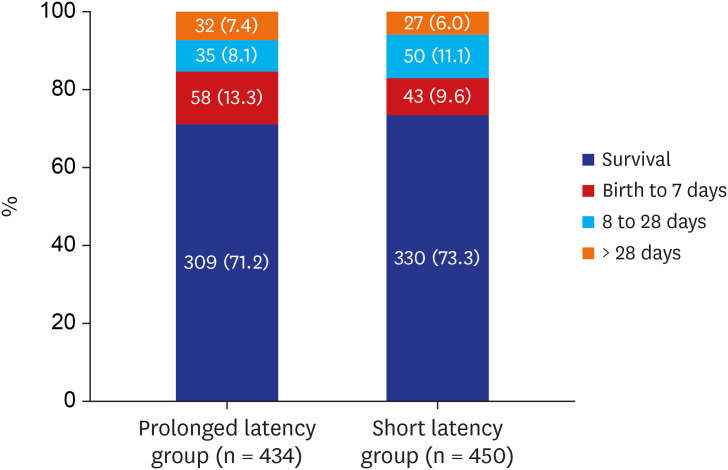
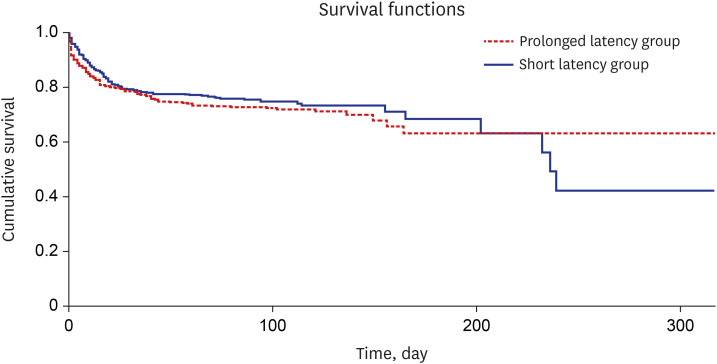




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download