1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. PMID:
30207593.

2. Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014; 50(7):1330–1344. PMID:
24650579.


3. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004; 291(2):187–194. PMID:
14722144.

4. Park HA, Nam SY, Lee SK, Kim SG, Shim KN, Park SM, et al. The Korean guideline for gastric cancer screening. J Korean Med Assoc. 2015; 58(5):373–384.

5. Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008; 38(4):259–267. PMID:
18344316.


6. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016; 388(10060):2654–2664. PMID:
27156933.


7. Nakagawa M, Kojima K, Inokuchi M, Kato K, Sugita H, Kawano T, et al. Patterns, timing and risk factors of recurrence of gastric cancer after laparoscopic gastrectomy: reliable results following long-term follow-up. Eur J Surg Oncol. 2014; 40(10):1376–1382. PMID:
24915857.


8. Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg. 2014; 219(4):664–675. PMID:
25154671.

9. Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002; 28(4):455–461. PMID:
12099659.


10. Pak KH, Hyung WJ, Son T, Obama K, Woo Y, Kim HI, et al. Long-term oncologic outcomes of 714 consecutive laparoscopic gastrectomies for gastric cancer: results from the 7-year experience of a single institute. Surg Endosc. 2012; 26(1):130–136. PMID:
21789641.


11. Hosokawa O, Kaizaki Y, Watanabe K, Hattori M, Douden K, Hayashi H, et al. Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy. 2002; 34(6):469–473. PMID:
12048630.


12. Jang HJ, Choi MH, Shin WG, Kim KH, Baek IH, Kim KO, et al. Is annual endoscopic surveillance necessary for the early detection of gastric remnant cancer in Korea? A retrospective multi-center study. Hepatogastroenterology. 2014; 61(133):1283–1286. PMID:
25436297.

13. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24(31):4991–4997. PMID:
17075117.


14. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376(9742):687–697. PMID:
20728210.


15. Lee SY, Lee JH, Hwang NC, Kim YH, Rhee PL, Kim JJ, et al. The role of follow-up endoscopy after total gastrectomy for gastric cancer. Eur J Surg Oncol. 2005; 31(3):265–269. PMID:
15780561.


16. Bilici A, Salman T, Oven Ustaalioglu BB, Unek T, Seker M, Aliustaoglu M, et al. The prognostic value of detecting symptomatic or asymptomatic recurrence in patients with gastric cancer after a curative gastrectomy. J Surg Res. 2013; 180(1):e1–9. PMID:
22520575.

17. Peixoto RD, Lim HJ, Kim H, Abdullah A, Cheung WY. Patterns of surveillance following curative intent therapy for gastroesophageal cancer. J Gastrointest Cancer. 2014; 45(3):325–333. PMID:
24756830.


18. Park CH, Park JC, Chung H, Shin SK, Lee SK, Cheong JH, et al. Impact of the surveillance interval on the survival of patients who undergo curative surgery for gastric cancer. Ann Surg Oncol. 2016; 23(2):539–545. PMID:
26424325.


19. Eom BW, Ryu KW, Lee JH, Choi IJ, Kook MC, Cho SJ, et al. Oncologic effectiveness of regular follow-up to detect recurrence after curative resection of gastric cancer. Ann Surg Oncol. 2011; 18(2):358–364. PMID:
21042946.


20. Cardoso R, Coburn NG, Seevaratnam R, Mahar A, Helyer L, Law C, et al. A systematic review of patient surveillance after curative gastrectomy for gastric cancer: a brief review. Gastric Cancer. 2012; 15(Suppl 1):S164–S167. PMID:
22382929.

21. Park SJ, Park YS, Jung IS, Yoon H, Shin CM, Ahn SH, et al. Is endoscopic surveillance necessary for patients who undergo total gastrectomy for gastric cancer? PLoS One. 2018; 13(6):e0196170. PMID:
29856747.

22. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17(12):3077–3079. PMID:
20882416.


23. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021; 24(1):1–21. PMID:
32060757.

24. Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019; 39(1):10. PMID:
30885279.


25. De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017; 20(1):20–30.

26. Baiocchi GL, Kodera Y, Marrelli D, Pacelli F, Morgagni P, Roviello F, et al. Follow-up after gastrectomy for cancer: results of an international web round table. World J Gastroenterol. 2014; 20(34):11966–11971. PMID:
25232232.



27. Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19(1):1–48. PMID:
30944757.


28. Hur H, Song KY, Park CH, Jeon HM. Follow-up strategy after curative resection of gastric cancer: a nationwide survey in Korea. Ann Surg Oncol. 2010; 17(1):54–64. PMID:
19777193.


29. Han ES, Seo HS, Kim JH, Lee HH. Surveillance endoscopy guidelines for postgastrectomy patients based on risk of developing remnant gastric cancer. Ann Surg Oncol. 2020; 27(11):4216–4224. PMID:
32372310.


30. Greene FL. Management of gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg. 1996; 223(6):701–706. PMID:
8645043.



31. Fujita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T. Relationship between the histological type of initial lesions and the risk for the development of remnant gastric cancers after gastrectomy for synchronous multiple gastric cancers. World J Surg. 2010; 34(2):296–302. PMID:
20012285.


32. Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007; 94(1):92–95. PMID:
17054314.


33. Na HK, Ahn JY, Lee JH, Kim DH, Choi KD, Song HJ, et al. Clinical outcomes of recurrent gastric cancer detected by upper endoscopy after curative total gastrectomy. Tumori. 2017; 103(2):164–169. PMID:
27841437.


34. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87(2):236–242. PMID:
10671934.


35. Barakat M, Seif M, Abdelfatah MM, Ofosu A, Carr-Locke DL, Othman MO. Endoscopic submucosal dissection for early neoplastic lesions in the surgically altered stomach: a systematic review and meta-analysis. Surg Endosc. 2019; 33(8):2381–2395. PMID:
30963259.


36. Yoo CH, Sohn BH, Han WK, Pae WK. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004; 36(1):50–55. PMID:
20396565.



37. Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009; 146(3):469–474. PMID:
19715803.


38. Bar-Sela G, Tsalic M, Steiner M, Wollner M, Haim N. Local recurrence following adjuvant chemotherapy without radiotherapy in completely resected stomach and gastroesophageal junction adenocarcinoma. Anticancer Res. 2009; 29(5):1853–1856. PMID:
19443416.

39. Papachristou DN, Karas M, Fortner JG. Anastomotic recurrence in the oesophagus complicating gastrectomy for adenocarcinoma of the stomach. Br J Surg. 1979; 66(9):609–612. PMID:
497643.


40. Nishimura M, Honda I, Watanabe S, Nagata M, Souda H, Miyazaki M. Recurrence in jejunal pouch after proximal gastrectomy for early upper gastric cancer. Gastric Cancer. 2003; 6(3):197–201. PMID:
14520535.


41. Namikawa T, Kobayashi M, Okamoto K, Okabayashi T, Akimori T, Sugimoto T, et al. Recurrence of gastric cancer in the jejunal pouch after completion gastrectomy. Gastric Cancer. 2007; 10(4):256–259. PMID:
18095082.


42. Lee MS, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013; 27(7):2598–2605. PMID:
23539255.


43. Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013; 23(4):323–331. PMID:
23379920.


44. D'Ugo D, Biondi A, Tufo A, Persiani R. Follow-up: the evidence. Dig Surg. 2013; 30(2):159–168. PMID:
23867593.

45. Aurello P, Petrucciani N, Antolino L, Giulitti D, D'Angelo F, Ramacciato G. Follow-up after curative resection for gastric cancer: is it time to tailor it? World J Gastroenterol. 2017; 23(19):3379–3387. PMID:
28596674.



46. Nilsson M. Postgastrectomy follow-up in the West: evidence base, guidelines, and daily practice. Gastric Cancer. 2017; 20(Suppl 1):135–140. PMID:
27718134.


47. Bettmann MA, Heeren T, Greenfield A, Goudey C. Adverse events with radiographic contrast agents: results of the SCVIR Contrast Agent Registry. Radiology. 1997; 203(3):611–620. PMID:
9169677.


48. ASGE Standards of Practice Committee. Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012; 76(4):707–718. PMID:
22985638.

49. Best LM, Mughal M, Gurusamy KS. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016; 3(3):CD011389. PMID:
27030300.

50. Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013; 27(5):1509–1520. PMID:
23263644.



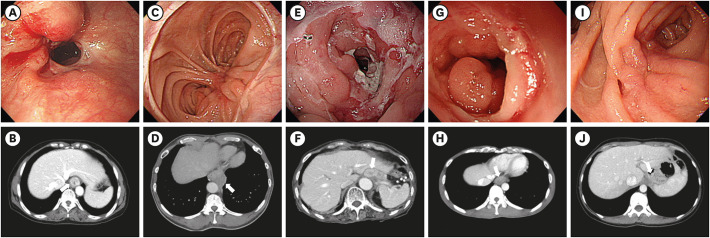
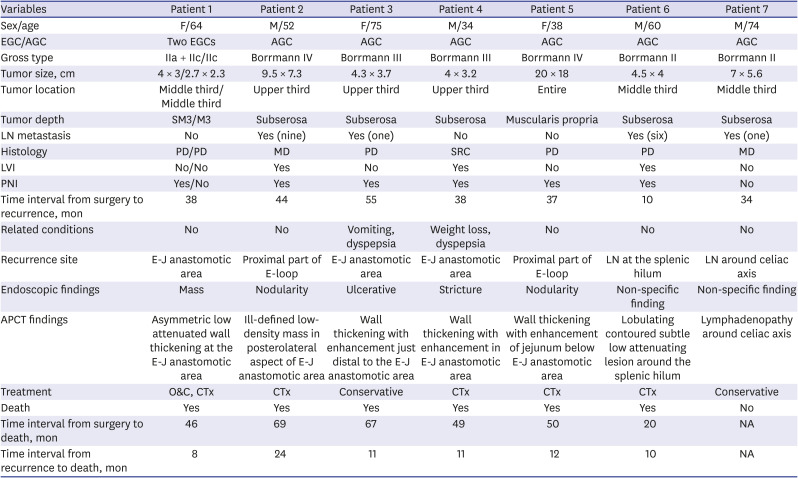
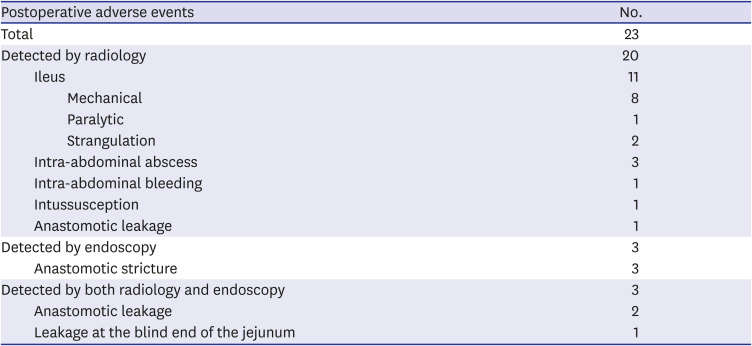
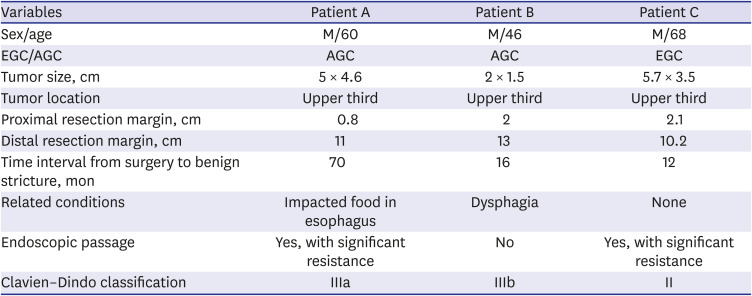




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download