This article has been corrected. See "Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes" in Volume 45 on page 796.
Abstract
The cardiovascular disease continuum begins with risk factors such as diabetes mellitus (DM), progresses to vasculopathy and myocardial dysfunction, and finally ends with cardiovascular death. Diabetes is associated with a 2- to 4-fold increased risk for heart failure (HF). Moreover, HF patients with DM have a worse prognosis than those without DM. Diabetes can cause myocardial ischemia via micro- and macrovasculopathy and can directly exert deleterious effects on the myocardium. Hyperglycemia, hyperinsulinemia, and insulin resistance can cause alterations in vascular homeostasis. Then, reduced nitric oxide and increased reactive oxygen species levels favor inflammation leading to atherothrombotic progression and myocardial dysfunction. The classification, diagnosis, and treatment of HF for a patient with and without DM remain the same. Until now, drugs targeting neurohumoral and metabolic pathways improved mortality and morbidity in HF with reduced ejection fraction (HFrEF). Therefore, all HFrEF patients should receive guideline-directed medical therapy. By contrast, drugs modulating neurohumoral activity did not improve survival in HF with preserved ejection fraction (HFpEF) patients. Trials investigating whether sodium-glucose cotransporter-2 inhibitors are effective in HFpEF are on-going. This review will summarize the epidemiology, pathophysiology, and treatment of HF in diabetes.
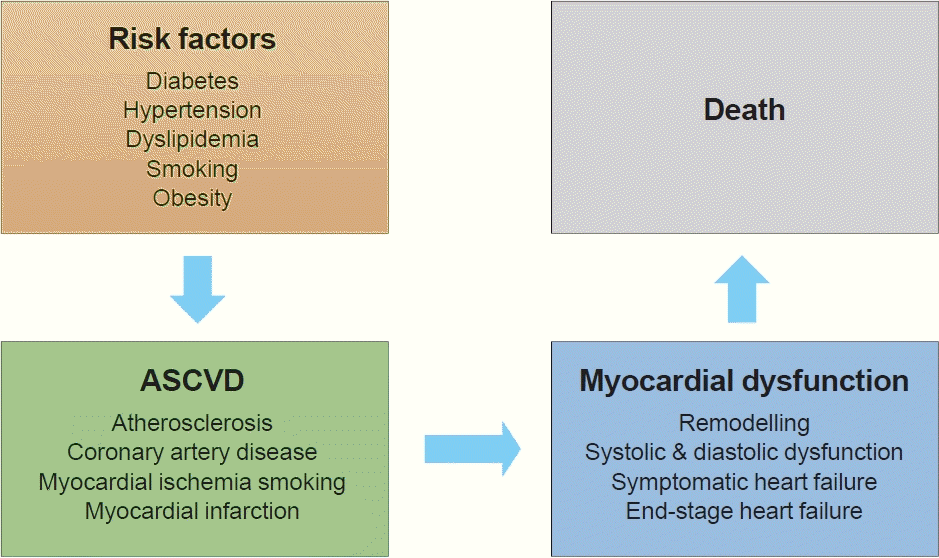
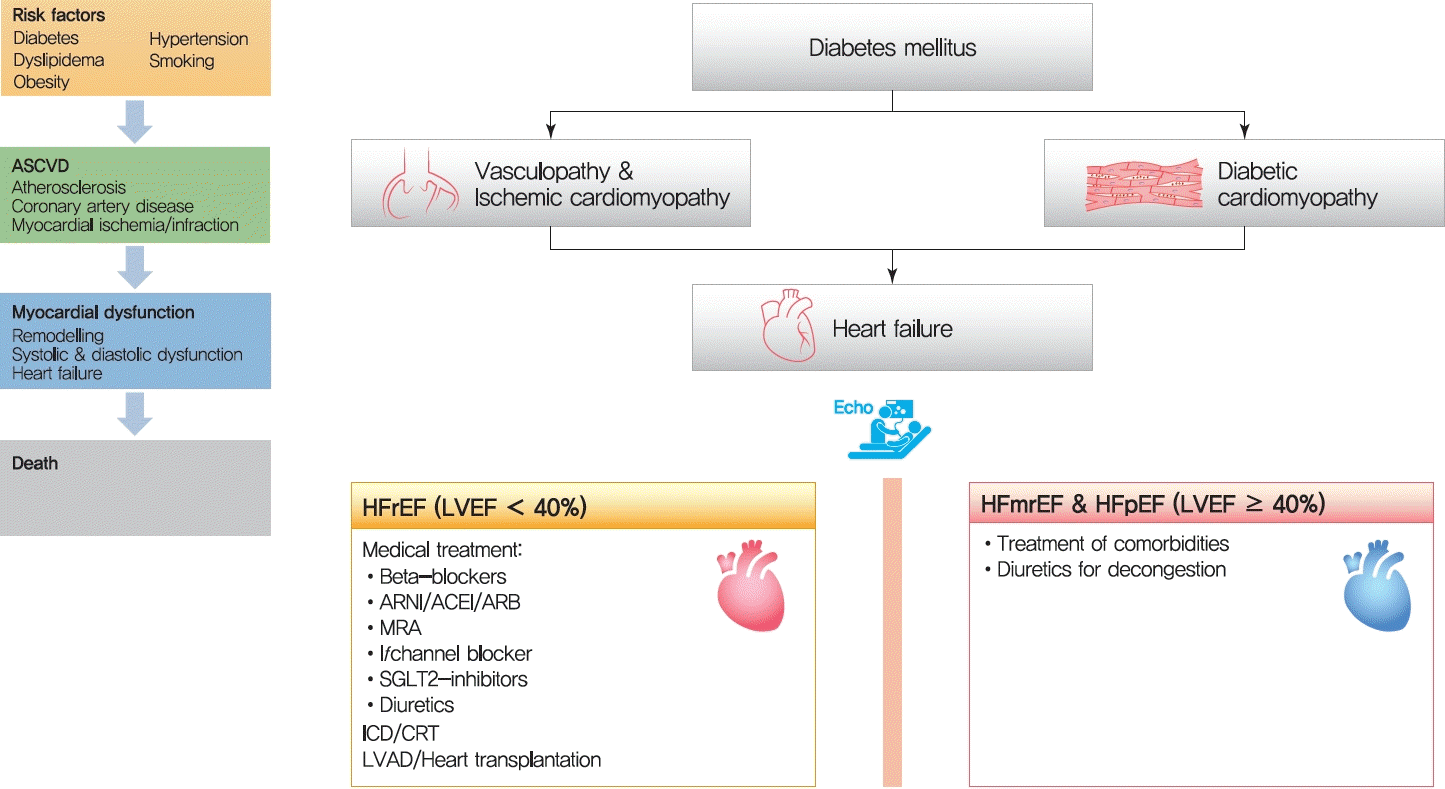
Thirty years ago, Dzau and Braunwald [1] introduced the cardiovascular disease continuum and framed cardiovascular disease as a chain of events, initiated by a myriad of related and unrelated risk factors and progressing through numerous physiological pathways and processes to the development of end-stage heart disease (Fig. 1) [2]. The cardiovascular disease continuum begins with diabetes mellitus (DM), hypertension, and dyslipidemia, among others, then results in advanced heart failure (HF) and cardiovascular death. The cardiovascular disease continuum also emphasizes the possibility that therapeutic intervention at every stage may prevent or slow the development of symptomatic HF and hopefully prolong life [2]. However, this concept has been untrue for diabetes because clinical trials showed that intensive glucose-lowering therapy did not translate into better clinical outcomes in diabetic patients [3-6].
Not all diabetic patients develop HF, and not all HF patients have diabetes [7,8]. Nonetheless, diabetes is an important risk factor for the development of HF. Diabetes increases the risk for HF [9-11] and complicates its course, such that HF patients with DM had worse outcomes than those without DM [12,13].
The scientific interest in HF in DM has increased significantly with the publications of recent cardiovascular outcome trials of newer antidiabetic drugs that robustly showed clinical benefit and altered the cardiovascular disease continuum for the first time [14,15]. This review will summarize the epidemiology, pathophysiology, and management of HF in diabetes from the viewpoint of a clinical cardiologist specialized in HF management.
Diabetes is a serious and increasing global health burden. The number of people with diabetes increased from 108 million in 1980 to 422 million in 2014, in which 8.5% of adults ≥18 years had diabetes. It is expected that over 592 million people worldwide will have diabetes by 2035. Regarding death, 1.6 million deaths were directly caused by diabetes in 2016 [16,17].
According to the diabetes fact sheet in Korea 2020, the prevalence of diabetes among adults 30 years or older was 13.8%, representing approximately 4.94 million Koreans in 2020. The prevalence was 27.6% among adults aged ≥65 years. Approximately 61.3% of patients with DM have coexisting hypertension, and in patients aged ≥65 years with DM, 74.3% also had hypertension. DM and hypertension are independent risk factors for HF, and their coexistence predisposes excess cumulative risk for HF development [18].
Diabetes is associated with a 2- to 4-fold increased risk of HF. In the Framingham Heart Study, DM was associated with a nearly 2-fold increase in the risk of incident HF in men and a 4-fold increase in women, even after adjustment for other cardiovascular risk factors [10]. In patients with known coronary artery disease in the Heart and Soul Study, DM was also associated with a 3.3-fold higher adjusted risk of incident HF [19]. Hence, DM is an important risk factor that promotes the progression of each stage in the cardiovascular disease continuum. Other risk factors for incident HF include older age, longer diabetes duration, ischemic heart disease, greater body weight, and higher creatinine level, among others; therefore,patients with multiple risk factors should receive special medical attention [20].
Among HF patients, the prevalence of DM is 2 to 2.5 times higher than in the general population. In the Korean Heart Failure (KorHF) registry from 1998 to 2003, the prevalence of DM was 31% [21]. In the Korean Acute Heart Failure Registry (KorAHF) from 2004 to 2009, 36% had diabetes [7]. These are similar to the prevalence of DM in Europe (33%) in the EuroHeart Failure Survey II (EHFS II) [22] but found to be less than that of the United States, with 44% according to the Acute Decompensated Heart Failure National Registry (ADHERE) [23]. Interestingly, only 11% of African HF patients had diabetes in the Sub-Saharan Africa Survey of Heart Failure (THESUS-HF) registry [24].
HF patients with DM had more unfavorable characteristics compared to those without DM. They have more ischemic etiology, higher body mass index, heart rate, systolic blood pressure, creatinine level, and N-terminal pro-B-type natriuretic peptide level than those without DM, which may explain the worse clinical outcomes of these patients [25].
The exact pathophysiologic mechanism linking diabetes and HF is unknown. Hyperglycemia, insulin resistance, and hyperinsulinemia all seem to initiate and perpetuate disease progression. Diabetes can cause myocardial ischemia via vasculopathy (both micro- and macrovasculopathy) and can directly exert deleterious effects on the myocardium (cardiac myocyte and interstitium).
Prolonged exposure to hyperglycemia causes vasculopathy, and there exists a linear relationship between glucose level and its detrimental effects. The concept of the “glycemic continuum” reflects that the negative effects of hyperglycemia occur even at levels below the threshold for DM diagnosis [26,27]. Both micro- and macrovasculopathy are the principal causes of morbidity and mortality in diabetic patients. Regarding myocardial perfusion, DM causes clinical and subclinical ischemia via both macro- and microvasculopathy, leading to myocardial dysfunction.
In the center of vasculopathy, the alteration of endothelial and vascular smooth muscle cells (VSMCs) plays a key role. Under normal conditions, the endothelium constitutively produces nitric oxide (NO) via endothelial NO synthase [28]. NO causes vasodilatation via activation of guanylyl cyclase in VSMCs. It also inhibits the proliferation and migration of VSMCs, thus inhibiting the atherosclerotic process [29-31]. In contrast, the loss of NO increases pro-inflammatory activity via activation of nuclear factor kappa B (NF-κΒ); expression of leukocyte adhesion molecules and production of chemokines and cytokines [31], which promotes monocyte and VSMC migration into the intima and formation of macrophage foam cells.
The bioavailability of NO is determined by its synthase and degradation. Its level also reflects vascular health. In patients with diabetes, hyperglycemia, free fatty acids [32-34], and insulin resistance [35,36], increased reactive oxygen species (ROS) activate protein kinase C, leading to low NO levels [37-39] and endothelial dysfunction. These alterations in vascular homeostasis due to endothelial and VSMC dysfunction favor a pro-inflammatory/thrombotic state, which ultimately leads to atherothrombosis.
At the level of the capillaries, abnormal intercellular signaling in endothelial cells decreases the capillary diameter and induces microvascular rarefaction in diabetic conditions in a human in vitro model of angiogenesis and in mice [40,41]. In diabetic porcine models, DM caused alterations in capillary structures at 2 months after DM induction and myocardial hypoperfusion before the development of significant epicardial coronary artery stenosis (Supplementary Fig. 1) [42].
Coronary circulation consists of epicardial coronary arteries, arterioles, and capillaries. Myocardial perfusion occurs during the diastole of each cardiac cycle following the pressure gradient between the epicardial coronary artery and left ventricular (LV) diastolic pressure; the distribution of blood flow then matches the dynamic needs of local tissue metabolism, which is regulated by arterioles and capillaries [43,44]. Because myocardial oxygen extraction is near-maximal at rest, myocardial oxygen delivery is almost dependent on coronary blood flow. In diabetic patients, accelerated atherosclerosis with luminal narrowing in the epicardial coronary artery was believed to be the main mechanism for the insufficient blood supply. However, many diabetic patients have “paradoxical” absence of significant stenosis in epicardial arteries with significant ischemic symptoms and positive exercise test [45-47], implying coronary microvasculopathy [48,49].
The coronary flow reserve (CFR) is the ratio between hyperemic and resting coronary flow, and the reduction of CFR has been reported to represent coronary microvascular dysfunction [50]. A reduction in CFR and myocardial blood flow was associated with increased cardiac mortality in diabetic patients without coronary artery stenosis [51-54].
Diabetic cardiomyopathy is defined by the existence of abnormal myocardial structure and performance in the absence of other cardiac risk factors, such as coronary artery disease, hypertension, and significant valvular disease, in individuals with DM [55]. Although the exact mechanisms need to be elucidated, several mechanisms have been proposed for the development of diabetic cardiomyopathy.
Hyperglycemia causes the formation of advanced glycation end products (AGEs), which are glycated proteins or lipids after prolonged exposure to glucose. AGEs can cross-link with extracellular matrix proteins, increase fibrosis, and impair myocardial relaxation [56,57]. AGEs can also cause intracellular damage via activation of the receptors for AGEs, leading to an increase in cytosolic ROS and activation of inflammatory pathways via NF-κΒ signaling [58,59].
ROS can mediate mitochondrial uncoupling and reduce cardiac energy efficiency [60]. Impairment in mitochondrial bioenergetics results in impaired intracellular calcium handling. Calcium reuptake via sarcoendoplasmic reticulum calcium transport ATPase (SERCA)-2 into the sarcoplasmic reticulum is an energy-dependent process and may result in both contraction and relaxation abnormalities [61]. Impaired glucose tolerance and increased fatty acid uptake by cardiac myocytes may exceed mitochondrial oxidative capacity, leading to lipid over-storage and production of lipotoxic metabolites and ROS (Fig. 2) [62,63].
There exists a chronic activation of the renin-angiotensin-aldosterone system in diabetic patients. Increased angiotensin II (AT-II) level causes vasoconstriction and increases the afterload and promote LV hypertrophy. AT-II also promotes collagen production, extracellular matrix protein accumulation leading to myocardial contractile dysfunction [64].
Oxidative stress, inflammation, impaired mitochondrial energetics, intracellular calcium handling, and increased neurohumoral activation all contribute to the anatomic and functional alteration associated with diabetic cardiomyopathy.
For diagnosis of HF, the patients should have typical symptoms (e.g., shortness of breath, fatigue) and signs (e.g., pulmonary and ankle edema) of HF, and objective evidence of functional and structural cardiac abnormality leading to reduced cardiac output and/or increased intracardiac filling pressure [65]. Currently, HF types are further classified according to the left ventricular ejection fraction (LVEF) and defined as HF with reduced ejection fraction (HFrEF) (LVEF<40%), HF with mid-range ejection fraction (HFmrEF) (40%≤ LVEF <50%), and HF with preserved ejection fraction (HFpEF; LVEF ≥50%) [65]. For diagnosis of HFmrEF and HFpEF, the patients should additionally have elevated levels of natriuretic peptides and either relevant structural heart disease such as LV hypertrophy and/or left atrial enlargement and/or diastolic dysfunction (Fig. 3A) [65]. In the KorAHF registry, 59.1%, 15.8%, and 25.1% had HFrEF, HFmrEF, and HFpEF, respectively [66]. Among patients with diabetes 64%, 14.4%, and 21.6% had HFrEF, HFmrEF, and HFpEF, respectively.
The diagnosis of HFpEF is challenging. Since HFpEF diagnosis based on echocardiographic data and natriuretic peptide levels has limited sensitivity, revised algorithms with scoring systems have been proposed recently.
Reddy et al. [17] proposed a H2FPEF score which consists of six dichotomized variables: Heavy (a body mass index >30 kg/m2, 2 points); Hypertension (use of ≥2 antihypertensive medications, 1 point); atrial Fibrillation (3 points); Pulmonary hypertension (pulmonary artery systolic pressure >35 mm Hg, 1 point); Elderly (age >60 years, 1 point); and Filling pressures (E/e´ >9, 1 point). The score ranges from 0 to 9 and at a score of ≥6, HFpEF was diagnosed with a probability ≥90%.
The Heart Failure Association algorithm of the ESC (HFA-PEFF) consists of (1) Pretest assessment, (2) diagnostic workup with Echocardiogram and natriuretic peptide score, (3) advanced workup with Functional testing in case of uncertainty, and (4) Final etiological workup [67]. In the calculation of the HPA-HEFF score, for each major criterion met, 2 points are awarded, whereas 1 point is awarded for a minor criterion. A score of ≥5 based on echocardiographic and natriuretic peptide levels is diagnostic of HFpEF. A score of ≤1 makes a diagnosis of HFpEF very unlikely.
Borlaug [68] proposed a practical approach to the diagnosis of HFpEF. After an initial clinical assessment of the patient’s history, symptoms, and signs, the risk score is calculated. Additional workup in the form of invasive and non-invasive diastolic stress test is recommended for patients with intermediate probability to confirm the HFpEF diagnosis (Fig. 3B).
HFrEF and HFpEF are characterized by different anatomy and degree of neurohumoral activation. Patients with HFrEF have an enlarged LV cavity and relatively small LV wall thickness, whereas patients with HFpEF have relatively normal LV diameter, but increased wall thickness [69]. According to the law of Laplace, wall tension correlates directly with the LV diameter and the pressure, but inversely with the wall thickness. Therefore, HFrEF patients have higher wall tension and higher natriuretic peptide levels that are secreted by ventricles in response to increased wall stress [70]. The two distinct HF types respond differently to the drugs that modulate neurohumoral activation.
Currently, there are seven classes of drugs that improved the clinical outcomes in HFrEF and they are (1) renin-angiotensin-system (RAS) inhibitors, (2) angiotensin receptor neprilysin inhibitor (ARNI), (3) mineralocorticoid receptor antagonist (MRA), (4) beta-blockers, (5) If -channel inhibitor, (6) sodium-glucose cotransporter-2 (SGLT2) inhibitors, and (7) soluble guanylate cyclase stimulator.
In the Studies of Left Ventricular Dysfunction (SOLVD) [71], enalapril 10 mg twice daily reduced the mortality in hospitalized HF patients with LVEF ≤35% compared to placebo. In the Valsartan Heart Failure Trial (Val-HeFT) with patients with LVEF <40%, valsartan 160 mg twice daily reduced the composite endpoint of mortality and morbidity, defined as the incidence of cardiac arrest with resuscitation, hospitalization for HF (HHF), or receipt of intravenous inotropic or vasodilator therapy compared to placebo by 13% (relative risk, 0.87; 97.5% confidence interval, 0.77 to 0.97; P=0.009) [72]. However, the morality was similar in the two groups. It is of note that angiotensin receptor blockers (ARBs) are reserved for patients who cannot tolerate angiotensin-converting enzyme inhibitors (ACEis).
ARNI consists of an ARB and a neprilysin inhibitor, an endopeptidase that degrades vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. A natriuretic peptide is considered the “natural antagonist” of angiotensin and has natriuretic, diuretic, vasodilatory, antifibrotic and antisympathetic effects [73]. In the Prospective Comparison of ARNI with ACEi to Determine Impact on Global Mortality and Morbidity in HF (PARADIGM-HF) study sacubitril-valsartan 200 mg twice daily reduced the composite of cardiovascular deaths and HHF by 20% (hazard ratio, 0.80; 95% confidence interval, 0.73 to 0.87; P<0.001) compared with enalapril 10 mg twice daily in optimally treated HFrEF patients with LVEF ≤40% [74].
In the randomized aldactone evaluation study (RALES), spironolactone 25 mg daily reduced morality by 30% (relative risk of death, 0.70; 95% confidence interval, 0.60 to 0.82; P<0.001) in patients with LVEF <35%. Electrolyte should be monitored regularly because hyperkalemia can occur.
The effect of beta-blockers in HFrEF seems to be substratespecific. Currently, three beta-blockers, i.e., carvedilol [75], bisoprolol [76], and metoprolol succinate [77] showed a beneficial effect in HFrEF. It is of note that beta-blockers have not been tested in acute HF, and in some meta-analyses their effect was neutral in patients with AF [78].
Low heart rate is associated with better survival. Ivabradine blocks If channel in the sinus node and slows heart rate without exerting a negative inotropic effect. In the Systolic Heart failure treatment with the IF inhibitor ivabradine Trial (SHIFT) [79], ivabradine reduced the composite of all-cause mortality and HHF by 20% in patients with LVEF ≤35% in sinus rhythm and with a heart rate >70 beats/min. In patients with heart rate >75 beats/min ivabradine also showed survival benefit [80].
SGLT2-inhibitors block glucose reuptake in the proximal tubules. In cardiovascular outcome studies in diabetic patients with and without atherosclerotic cardiovascular disease, empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin reduced all HHF [81-85]. In patients with HFrEF, both dapagliflozin and empagliflozin reduced the composite of all-cause death or HHF by approximately 25% and HHF by approximately 30% [86]. This effect was consistent in those with and without DM [25].
In VerICiguaT GlObal Study in Subjects With Heart Failure With Reduced EjectIon FrAction (VICTORIA) study [87], vericiguat, an oral soluble guanylate cyclase stimulator that improves the cardiac contractility, reduced the composite of all-cause deaths and HHF by 10% (hazard ratio, 0.90; 95% confidence interval, 0.82 to 0.98; P=0.02) in severe HF patients with LVEF <45%.
With the accumulation of clinical evidence, the algorithm for pharmacologic treatment has been changing (Fig. 4) [88]. The latest expert consensus recommends the initial use of beta-blockers and ARNI/ACEi/ARB. ARNI is preferred over ACEi or ARB. Each drug should be up titrated to the maximum tolerated dose every 2 weeks (Table 1). The addition of MRA, SGLT2-inhibitors, and ivabradine should also be considered in appropriate patients. Diuretics may be added and up titrated to achieve decongestion. Regular monitoring for renal function, electrolytes imbalance, and cardiac function is required.
HF patients are at increased risk for sudden cardiac death which is mainly caused by ventricular arrhythmias, bradycardia, and asystole. An implantable cardiac defibrillator (ICD) can treat bradycardia and potentially lethal ventricular arrhythmia. ICD is recommended for primary prevention in symptomatic HF patients with LVEF ≤35%, despite >3 months of optimal medical therapy (OMT).
Some HF patients show dyssynchronous LV contraction resulting ineffective translation of the LV contraction into stroke volume. Cardiac resynchronization therapy (CRT) is a modality of cardiac pacing that simultaneously paces the ventricles and restores the ventricular synchrony. It has been shown to improve symptoms and reduce morbidity and mortality. CRT is recommended for symptomatic patients with HF in sinus rhythm with a QRS duration >130 ms and with LVEF ≤35% despite OMT.
Since HFmrEF patients were generally included in HFpEF trials, the treatment for HFmrEF and HFpEF are considered the same. Until now, no drugs in HFrEF improved survival in HFpEF [89-92], although some may reduce the HHF. Despite the different response to medical therapy, interestingly, HFrEF and HFpEF have a similar prognosis [93]. Cardiovascular deaths remain the main cause of mortality in HF; however, the proportion of non-cardiovascular deaths is higher in HFpEF (30% to 40%) than HFrEF (15% to 20%) [94]. Therefore, the current practice guidelines emphasize the treatment of underlying disease. In HFpEF patients with congestion, diuretics can alleviate symptoms of HF.
Regarding SGLT2-inhibitors, sotagliflozin, an SGLT1 and 2 dual blocker reduced a composite of cardiovascular deaths and hospitalizations and urgent visits for HF in patients with diabetes and recent worsening HF, regardless of LVEF in the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) study [95]. However, the early termination of the trial and the small sample size of this subgroup made it difficult to draw any firm conclusion. Designated trials are on-going to evaluate the benefit of SGLT2 inhibitors in HFpEF patients [96,97].
Diabetes is an important risk factor for HF. Prolonged hyperglycemia, hyperinsulinemia, and insulin resistance can cause alterations in vascular homeostasis with reduced NO and increased ROS levels, which activate pro-inflammatory pathways that lead to atherothrombotic progression and myocardial dysfunction. HF patients with DM have worse prognosis than those without DM. The classification, diagnosis and treatment of HF remain the same for patients with and without DM. Until now, drugs targeting neuro-humoral and metabolic pathways improved mortality and morbidity in HFrEF, but not in HFpEF. Thus, all HFrEF patients should receive guideline-directed medical therapy.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0282.
Supplementary Fig. 1.
Vascular changes in the diabetic pigs. Optical coherence tomography images of the epicardial coronary arteries of diabetic and control pigs at 8 weeks showed no difference in lumen diameter. By contrast, diabetic pigs had a significantly smaller capillary diameter, more irregularity and disconnection. In cardiac magnet resonance imaging, diabetic pigs had lower myocardial perfusion suggesting that the microvasculopathy is the main cause for decreased myocardial perfusion at the early stages of diabetes.
REFERENCES
1. Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991; 121(4 Pt 1):1244–63.


2. Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes. Part I: pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006; 114:2850–70.

3. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in noninsulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998; 316:823–8.



4. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–59.



5. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–72.

6. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–39.

7. Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017; 47:341–53.



8. Park JJ, Choi DJ. Current status of heart failure: global and Korea. Korean J Intern Med. 2020; 35:487–97.



9. Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000; 35:1628–37.


10. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979; 241:2035–8.


11. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004; 27:1879–84.


12. From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006; 119:591–9.


13. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008; 29:1377–85.


14. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.



15. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.


16. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014; 103:137–49.


17. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018; 138:861–70.



18. Korean Diabetes Association. Diabetes fact sheet in Korea 2020. Seoul: Korean Diabetes Association;2020.
19. van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010; 33:2084–9.


20. Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001; 24:1614–9.


21. Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean Heart Failure registry. Korean Circ J. 2011; 41:363–71.



22. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006; 27:2725–36.


23. Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW; ADHERE Scientific Advisory Committee and Investigators. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007; 153:1021–8.

24. Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012; 172:1386–94.


25. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020; 323:1353–68.



26. Rutter MK, Nesto RW. Blood pressure, lipids and glucose in type 2 diabetes: how low should we go?: re-discovering personalized care. Eur Heart J. 2011; 32:2247–55.


27. Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality: the San Antonio Heart Study. Diabetes Care. 1998; 21:1167–72.


29. Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987; 148:1482–9.


30. Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991; 88:4651–5.



31. Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995; 76:980–6.


32. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999; 103:253–9.



33. Dichtl W, Nilsson L, Goncalves I, Ares MP, Banfi C, Calara F, et al. Very low-density lipoprotein activates nuclear factor- kappaB in endothelial cells. Circ Res. 1999; 84:1085–94.

34. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C: dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000; 49:1939–45.

35. Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996; 98:894–8.



36. Dixon AD. Should dental students be granted licensure automatically upon graduation?: a dental educator’s view. J Am Coll Dent. 1976; 43:221–8.

37. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000; 404:787–90.


38. Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001; 103:1618–23.


39. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998; 97:1695–701.

40. Yoon CH, Choi YE, Cha YR, Koh SJ, Choi JI, Kim TW, et al. Diabetes-induced jagged1 overexpression in endothelial cells causes retinal capillary regression in a murine model of diabetes mellitus: insights into diabetic retinopathy. Circulation. 2016; 134:233–47.

41. Yoon CH, Choi YE, Koh SJ, Choi JI, Park YB, Kim HS. High glucose-induced jagged 1 in endothelial cells disturbs notch signaling for angiogenesis: a novel mechanism of diabetic vasculopathy. J Mol Cell Cardiol. 2014; 69:52–66.
42. Park JJ, Kim SH, Kim MA, Chae IH, Choi DJ, Yoon CH. Effect of hyperglycemia on myocardial perfusion in diabetic porcine models and humans. J Korean Med Sci. 2019; 34:e202.

44. Chilian WM. Coronary microcirculation in health and disease: summary of an NHLBI workshop. Circulation. 1997; 95:522–8.


45. Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967; 276:1063–6.


46. Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973; 32:375–6.


47. Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002; 346:1948–53.


48. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62:263–71.

49. Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2011; 34:749–51.



50. Dimitrow PP, Galderisi M, Rigo F. The non-invasive documentation of coronary microcirculation impairment: role of transthoracic echocardiography. Cardiovasc Ultrasound. 2005; 3:18.


51. Galderisi M, Capaldo B, Sidiropulos M, D’Errico A, Ferrara L, Turco A, et al. Determinants of reduction of coronary flow reserve in patients with type 2 diabetes mellitus or arterial hypertension without angiographically determined epicardial coronary stenosis. Am J Hypertens. 2007; 20:1283–90.


52. Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003; 41:1387–93.
53. Wang L, Jerosch-Herold M, Jacobs DR Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006; 47:565–72.

54. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012; 126:1858–68.



55. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122:624–38.


56. Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004; 63:582–92.

57. Stewart Coats AJ. Common co-morbidities in heart failure: diabetes, functional mitral regurgitation and sleep apnoea. Int J Heart Fail. 2019; 1:25–41.
58. Aragno M, Mastrocola R, Medana C, Catalano MG, Vercellinatto I, Danni O, et al. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology. 2006; 147:5967–74.

59. Piperi C, Goumenos A, Adamopoulos C, Papavassiliou AG. AGE/RAGE signalling regulation by miRNAs: associations with diabetic complications and therapeutic potential. Int J Biochem Cell Biol. 2015; 60:197–201.


60. Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007; 56:2457–66.


61. Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002; 51:1166–71.


62. McGavock JM, Victor RG, Unger RH, Szczepaniak LS; American College of Physicians and the American Physiological Society. Adiposity of the heart, revisited. Ann Intern Med. 2006; 144:517–24.


63. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000; 97:1784–9.



64. Connelly KA, Kelly DJ, Zhang Y, Prior DL, Martin J, Cox AJ, et al. Functional, structural and molecular aspects of diastolic heart failure in the diabetic (mRen-2)27 rat. Cardiovasc Res. 2007; 76:280–91.

65. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18:891–975.

66. Kim KJ, Cho HJ, Kim MS, Kang J, Kim KH, Kim D, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019; 1:4–24.
67. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019; 40:3297–317.


68. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020; 17:559–73.

69. Park CS, Park JJ, Mebazaa A, Lee HY, Kim KH, Yoo BS, et al. Response to beta-blockers and natriuretic peptide level in acute heart failure: analysis of data from the Korean Acute Heart Failure Registry. Clin Res Cardiol. 2020 Jun 25 [Epub]. https://doi.org/10.1007/s00392-020-01689-8.

70. Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy: evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995; 96:1280–7.



71. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991; 325:293–302.


72. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001; 345:1667–75.


74. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371:993–1004.


75. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996; 334:1349–55.

76. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomized trial. Lancet. 1999; 353:9–13.
77. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999; 353:2007–7.
78. Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, et al. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017; 69:2885–96.

79. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, DubostBrama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010; 376:875–85.


80. Bohm M, Borer J, Ford I, Gonzalez-Juanatey JR, Komajda M, Lopez-Sendon J, et al. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol. 2013; 102:11–22.


81. Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016; 374:1094.

82. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:2099.


83. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–57.


84. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–35.


85. Kato ET, Kimura T. Sodium-glucose co-transporters-2 inhibitors and heart failure: state of the art review and future potentials. Int J Heart Fail. 2020; 2:12–22.

86. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.

87. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382:1883–93.


88. Writing Committee, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, et al. 2021 Update to the 2017 ACC Expert Consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021; 77:772–810.

89. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359:2456–67.


90. Yamamoto K, Origasa H, Hori M; J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013; 15:110–8.


91. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370:1383–92.

92. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CS, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381:1609–20.

93. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355:251–9.


94. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017; 69:556–69.


95. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384:117–28.


96. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. Evaluation of the effects of sodium-glucose co- transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019; 21:1279–87.

Fig. 2.
Pathophysiology for heart failure development in diabetes. AGE, advanced glycation end product; RAGE, receptors for AGE; ROS, reactive oxygen species; NO, nitric oxide; SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system.
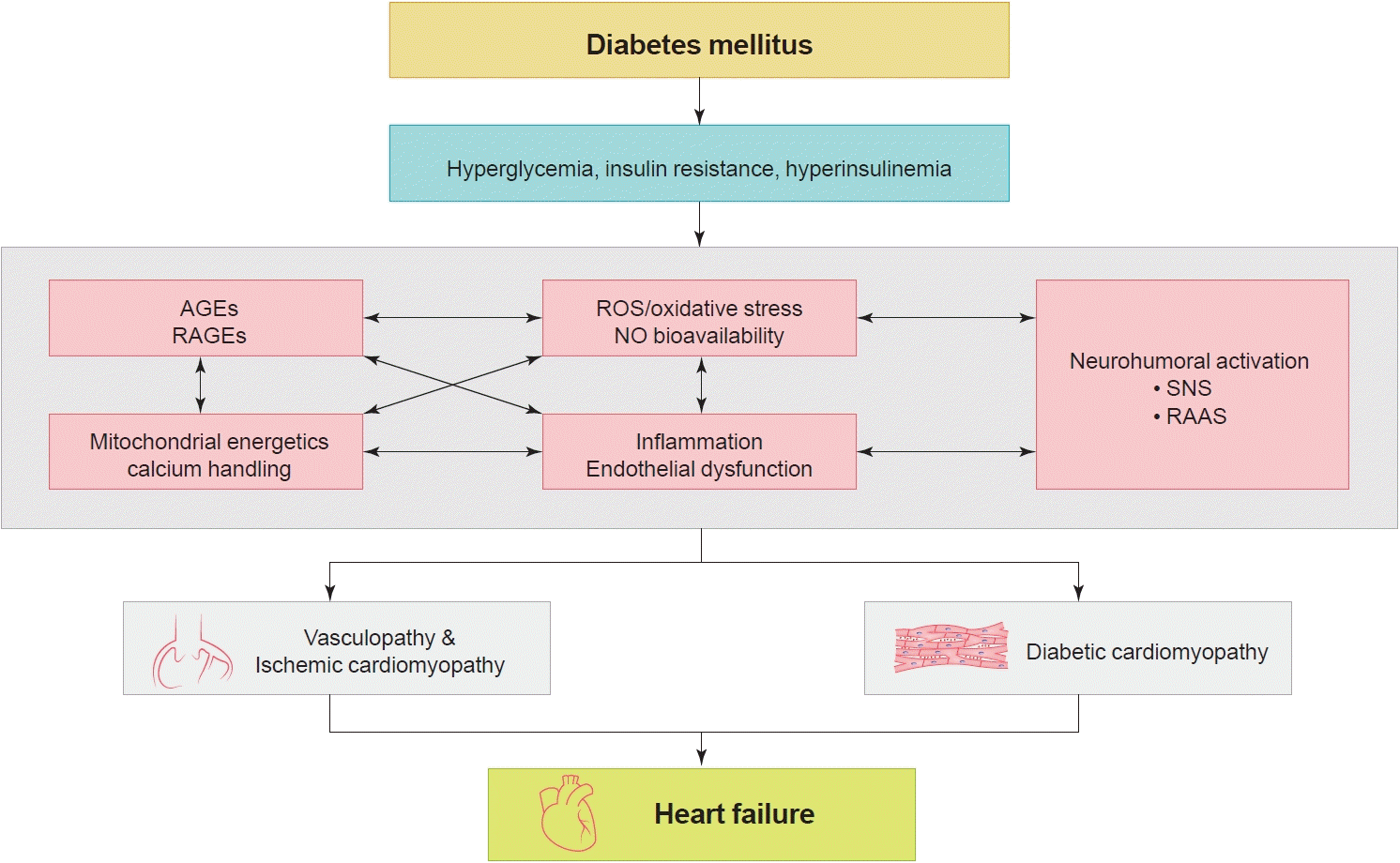
Fig. 3.
Diagnosis of heart failure. (A) Definition of heart failure types according to the ejection fraction. (B) Algorithm for the diagnosis of heart failure with preserved ejection fraction (HFpEF). HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; LVEF, left ventricular ejection fraction; H2FPEF, Heavy (a body mass index >30 kg/m2 , 2 points), Hypertension (use of ≥2 antihypertensive medications, 1 point), atrial Fibrillation (3 points), Pulmonary hypertension (pulmonary artery systolic pressure >35 mm Hg, 1 point), Elderly (age >60 years, 1 point), and Filling pressures (E/e´ >9, 1 point); HFA-PEFF, Heart Failure Association—Pretest assessment, (ii) diagnostic workup with Echocardiogram and natriuretic peptide score, (iii) advanced workup with Functional testing in case of uncertainty, and (iv) Final etiological workup; BMI, body mass index; LAVI, left atrial volume index; LVMI, left ventricular mass index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; BNP, B-type natriuretic peptide.
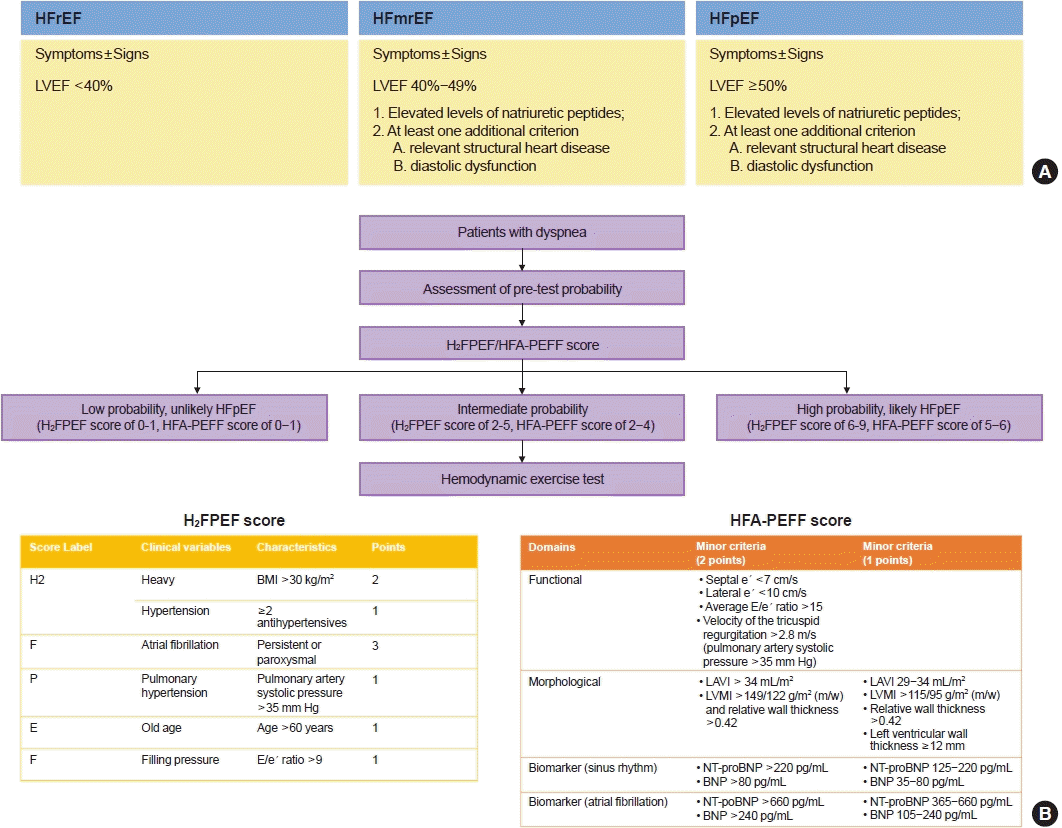
Fig. 4.
Treatment algorithm for guideline-directed medical therapy. HFrEF, heart failure with reduced ejection fraction; ARNI, angiotensin receptor-neprilysin inhibitor; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; eGFR, estimated glomerular filtration rate; NYHY, New York Heart Association; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium-glucose cotransporter-2; HR, heart rate.
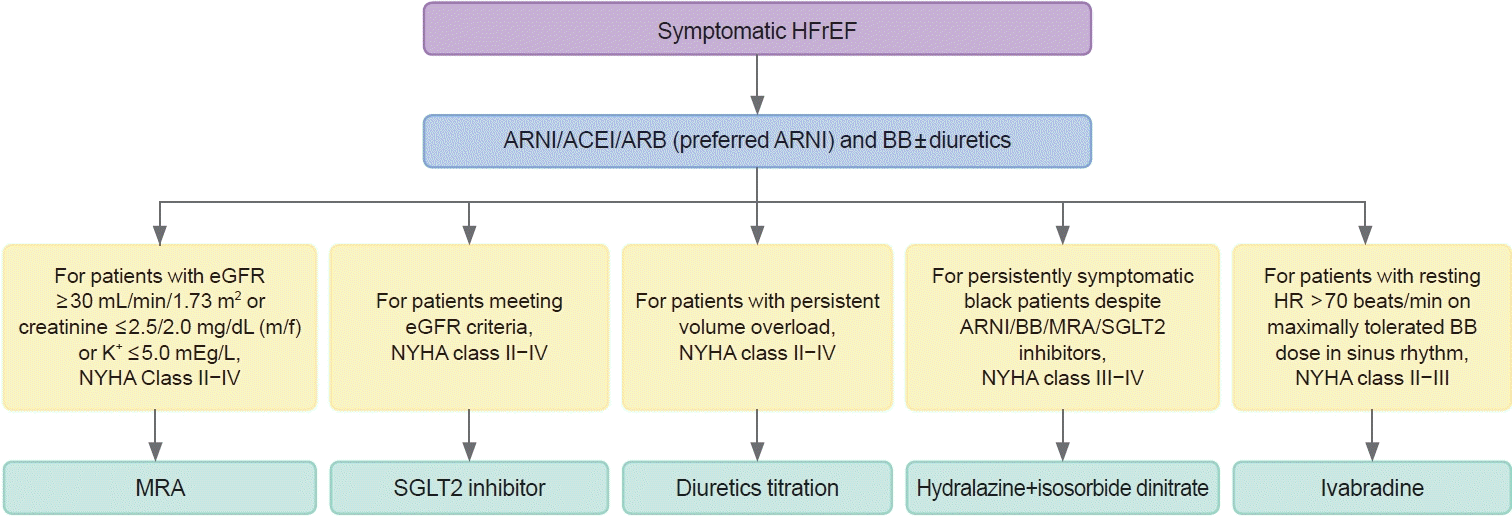
Table 1.
Guideline-directed medical therapy: drugs names and doses




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download