INTRODUCTION
METHODS
Study population
Measurements
Definition of DM and covariates
Statistical analysis
RESULTS
Baseline characteristics of the study population
Fig. 1
Flowchart of the study population. KNHANES, Korean National Health and Nutrition Examination Survey; DXA, dual-energy X-ray absorptiometry; BMI, body mass index.
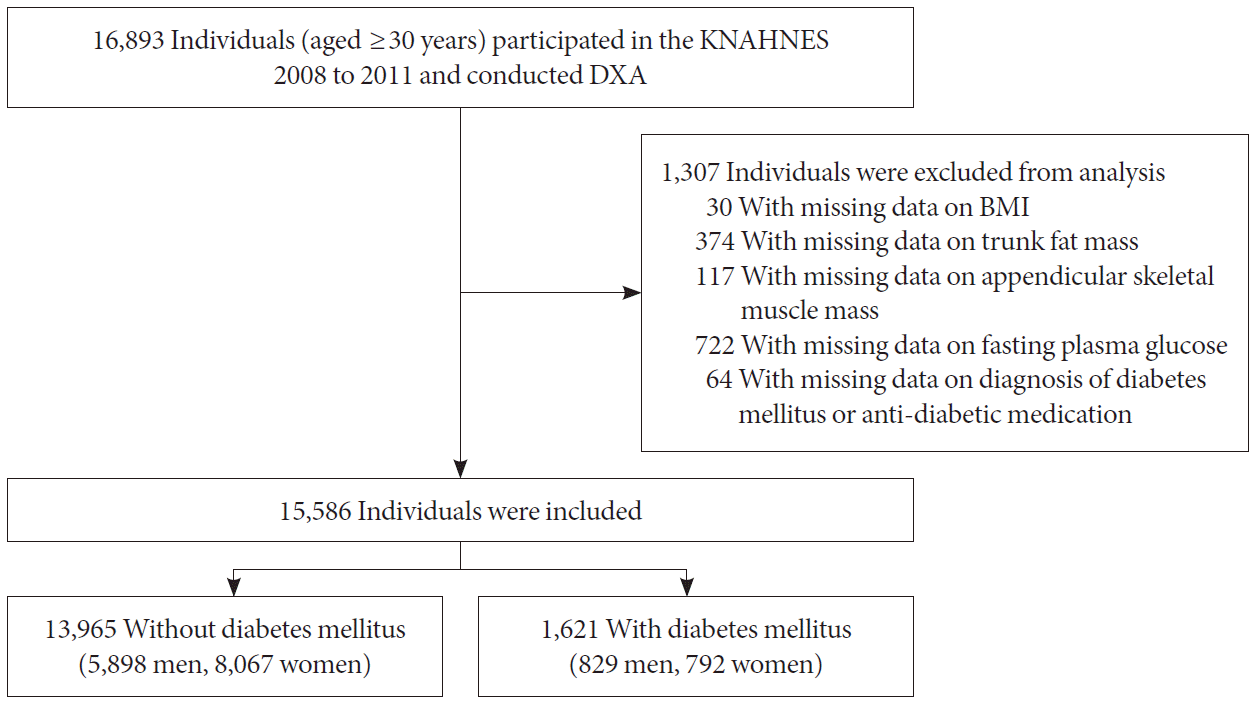
Table 1.
| Variable |
Men |
Women |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
30–49 yr |
50–69 yr |
≥70 yr |
30–49 yr |
50–69 yr |
≥70 yr |
|||||||
| No DM | DM | No DM | DM | No DM | DM | No DM | DM | No DM | DM | No DM | DM | |
| Number | 2,896 | 152 | 2,209 | 497 | 793 | 180 | 3,950 | 99 | 3,079 | 437 | 1,038 | 256 |
| Age, yr | 39.3±0.16 | 42.9±0.45c | 57.5±0.14 | 58.5±0.28b | 74.9±0.17 | 74.6±0.42 | 57.8±0.18 | 65.8±1.10c | 58.2±0.17 | 60.9±0.52c | 52.8±0.36 | 57.4±0.66c |
| WC, cm | 84.1±0.21 | 88.1±0.84c | 85.1±0.22 | 88.7±0.45c | 83.5±0.42 | 88.0±0.78c | 76.1±0.20 | 87.1±1.17c | 81.6±0.22 | 87.2±0.56c | 81.8±0.37 | 87.1±0.76c |
| BMI, kg/m2 | 24.3±0.07 | 25.6±0.33b | 23.9±0.07 | 24.9±0.18c | 22.7±0.13 | 23.9±0.27c | 22.9±0.07 | 26.5±0.41c | 24.2±0.06 | 25.5±0.19c | 23.6±0.13 | 25.2±0.25c |
| Trunk fat mass, kg | 16.0±0.13 | 17.6±0.52b | 14.8±0.14 | 16.0±0.28c | 13.9±0.23 | 16.4±0.45c | 18.7±0.12 | 23.0±0.76c | 20.0±0.12 | 21.2±0.29b | 17.9±0.24 | 20.4±0.44c |
| FMI, m2 | 0.35±0.003 | 0.39±0.01c | 0.34±0.003 | 0.37±0.005c | 0.33±0.005 | 0.39±0.009c | 0.39±0.002 | 0.48±0.013c | 0.43±0.003 | 0.47±0.005c | 0.40±0.004 | 0.46±0.008c |
| ASM, kg | 23.3±0.07 | 23.5±0.36 | 21.5±0.08 | 21.5±0.17 | 19.1±0.12 | 18.8±0.21 | 14.8±0.05 | 16.1±0.26c | 14.4±0.05 | 14.7±0.13b | 13.1±0.07 | 13.5±0.15b |
| MMI, m2 | 0.96±0.003 | 0.93±0.012b | 0.90±0.003 | 0.87±0.005c | 0.85±0.004 | 0.79±0.008c | 0.65±0.002 | 0.61±0.009c | 0.60±0.002 | 0.58±0.004c | 0.56±0.003 | 0.54±0.006b |
| SBP, mm Hg | 115.2±0.33 | 121.4±1.27c | 122.9±0.48 | 125.8±1.05a | 128.4±0.77 | 129.0±1.49 | 108.2±0.26 | 117.0±1.56c | 122.7±0.42 | 129.5±0.94c | 132.1±0.70 | 135.6±1.25a |
| DBP, mm Hg | 78.2±0.27 | 82.8±0.91c | 79.4±0.32 | 78.0±0.55a | 73.7±0.43 | 71.6±0.83a | 71.2±0.20 | 75.9±0.97c | 77.2±0.24 | 77.0±0.51 | 74.8±0.36 | 74.0±0.77 |
| Fasting glucose, mg/dL | 93.7±0.21 | 167.8±5.59c | 97.0±0.25 | 144.9±2.37c | 96.1±0.45 | 136.0±3.73c | 90.7±0.16 | 164.7±6.33c | 94.4±0.2 | 141.1±2.26c | 95.7±0.40 | 140.3±3.33c |
| TC, mg/dL | 192.4±0.73 | 196.3±3.76 | 190.7±0.89 | 180.0±2.16c | 180.2±1.34 | 181.3±3.72 | 180.9±0.62 | 195.7±4.82b | 202.3±0.86 | 195.6±2.30b | 200.0±1.31 | 198.3±2.94 |
| LDL-C, mg/dL | 115.9±0.65 | 110.6±3.44 | 114.7±0.86 | 101.1±1.87c | 109.2±1.24 | 109.6±3.40 | 109.7±0.55 | 116.0±4.54 | 126.8±0.73 | 117.2±2.12c | 125.7±1.17 | 120.4±2.53 |
| HDL-C, mg/dL | 45.8±0.25 | 43.3±0.98a | 45.8±0.30 | 42.4±0.56c | 45.6±0.49 | 42.0±0.94b | 51.8±0.21 | 47.0±0.99c | 49.9±0.27 | 46.1±0.69c | 46.2±0.41 | 44.9±0.81 |
| TG, mg/dL | 168.7±3.22 | 241.7±21.43b | 163.9±3.73 | 203.6±8.41c | 129.5±3.33 | 149.9±7.38a | 98.7±1.32 | 175.2±15.1c | 130.4±2.02 | 165.3±5.17c | 141.3±2.96 | 167.0±6.56b |
| AST, IU/L | 24.7±0.33 | 30.9±1.86b | 26.2±0.38 | 28.0±0.93 | 24.8±0.52 | 26.1±1.62 | 18.4±0.12 | 25.8±2.34b | 22.6±0.20 | 24.5±0.62b | 22.6±0.27 | 23.8±0.64 |
| ALT, IU/L | 29.4±0.54 | 40.2±2.47c | 25.2±0.40 | 29.5±0.97c | 19.5±0.45 | 23.9±1.28b | 15.8±0.20 | 29.7±4.00b | 20.3±0.30 | 25.5±0.86c | 17.0±0.30 | 21.1±0.76c |
| Low income | 695 (25.1) | 43 (33.3)a | 506 (23.1) | 139 (32.3)b | 184 (23.4) | 42 (22.8) | 982 (27) | 35 (41.2)b | 734 (24.7) | 110 (24.9) | 271 (26.3) | 52 (21.9) |
| Current smoker | 1,534 (54.1) | 85 (56.8) | 805 (38.6) | 177 (40.3) | 193 (24.6) | 41 (24.3) | 232 (6.6) | 3 (2.5) | 111 (4.1) | 14 (1.9)a | 65 (7.3) | 16 (7.7) |
| Drinking | 2,322 (80.1) | 117 (78.5) | 1,605 (74.2) | 347 (72.4) | 438 (56.5) | 90 (51.2) | 1,934 (49.2) | 46 (48) | 903 (31.8) | 93 (24.2)a | 168 (16.1) | 37 (16) |
| Regular exercise | 554 (19.6) | 35 (22.2) | 439 (20.9) | 88 (19.2) | 88 (11.1) | 20 (9.7) | 584 (15.2) | 13 (12.4) | 451 (15.1) | 44 (12.6) | 82 (7.7) | 22 (8.3) |
Values are presented as mean±standard error or number (%).
DM, diabetes mellitus; WC, waist circumference; BMI, body mass index; FMI, fat mass index; ASM, appendicular skeletal muscle; MMI, muscle mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
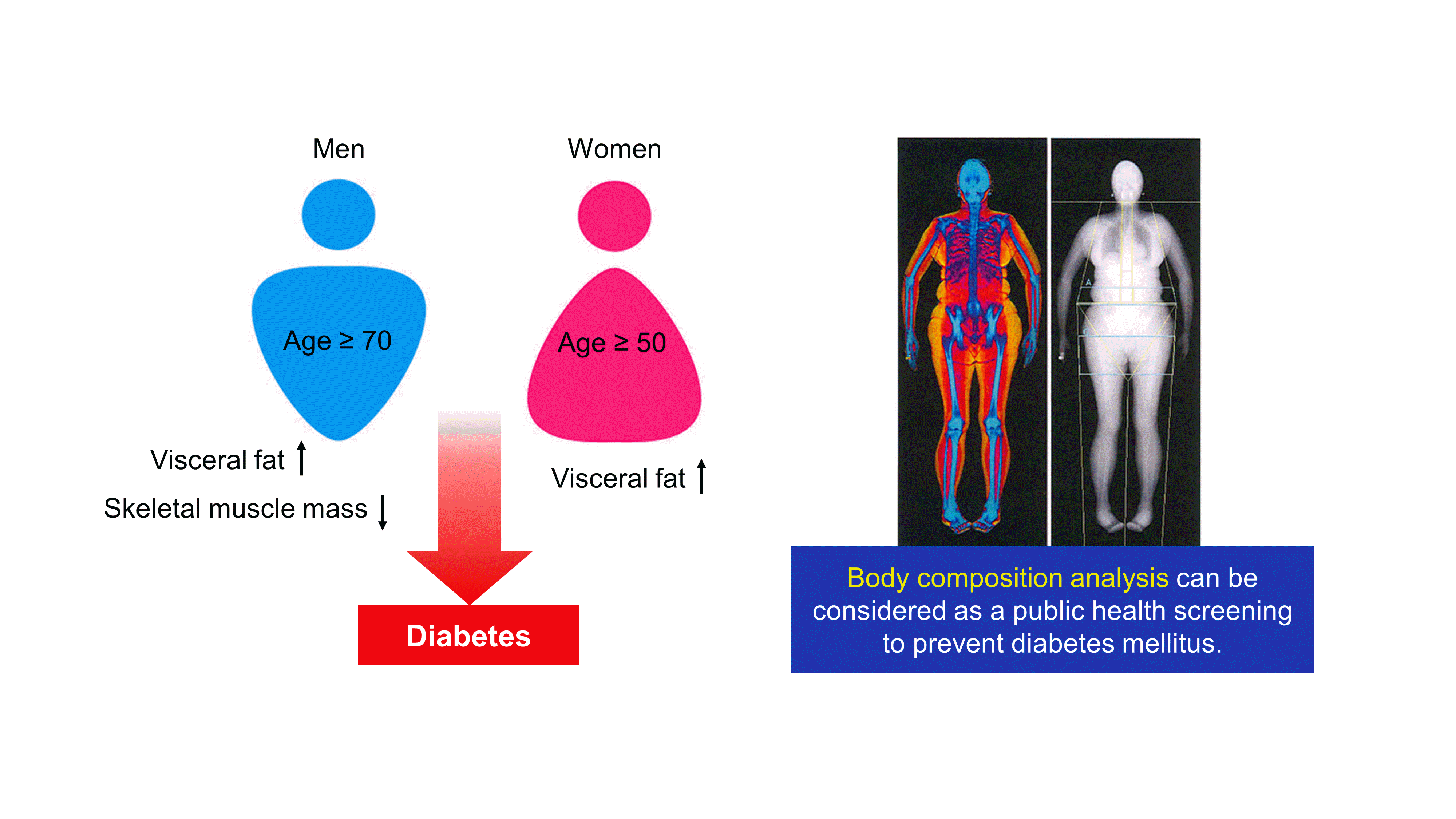
Age-related associations between body composition and DM in men and women after adjusting for age and BMI
Age-related associations between body composition and DM in men and women after adjusting for other metabolic parameters
Table 2.
| Variable |
30–49 yr |
50–69 yr |
≥70 yr |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | P value | Multivariate | P value | Univariate | P value | Multivariate | P value | Univariate | P value | Multivariate | P value | |
| Age, yr | 1.128 (1.09–1.168) | <0.001a | 1.123 (1.081–1.166) | <0.001a | 1.032 (1.014–1.051) | 0.001a | 1.007 (0.985–1.03) | 0.517 | 0.979 (0.925–1.037) | 0.473 | ||
| BMI, kg/m2 | 1.126 (1.064–1.192) | <0.001a | 1.049 (0.963–1.142) | 0.27 | 1.134 (1.085–1.186) | <0.001a | 1.094 (1.029–1.163) | 0.004a | 1.157 (1.085–1.234) | <0.001a | 1.03 (0.946–1.121) | 0.497 |
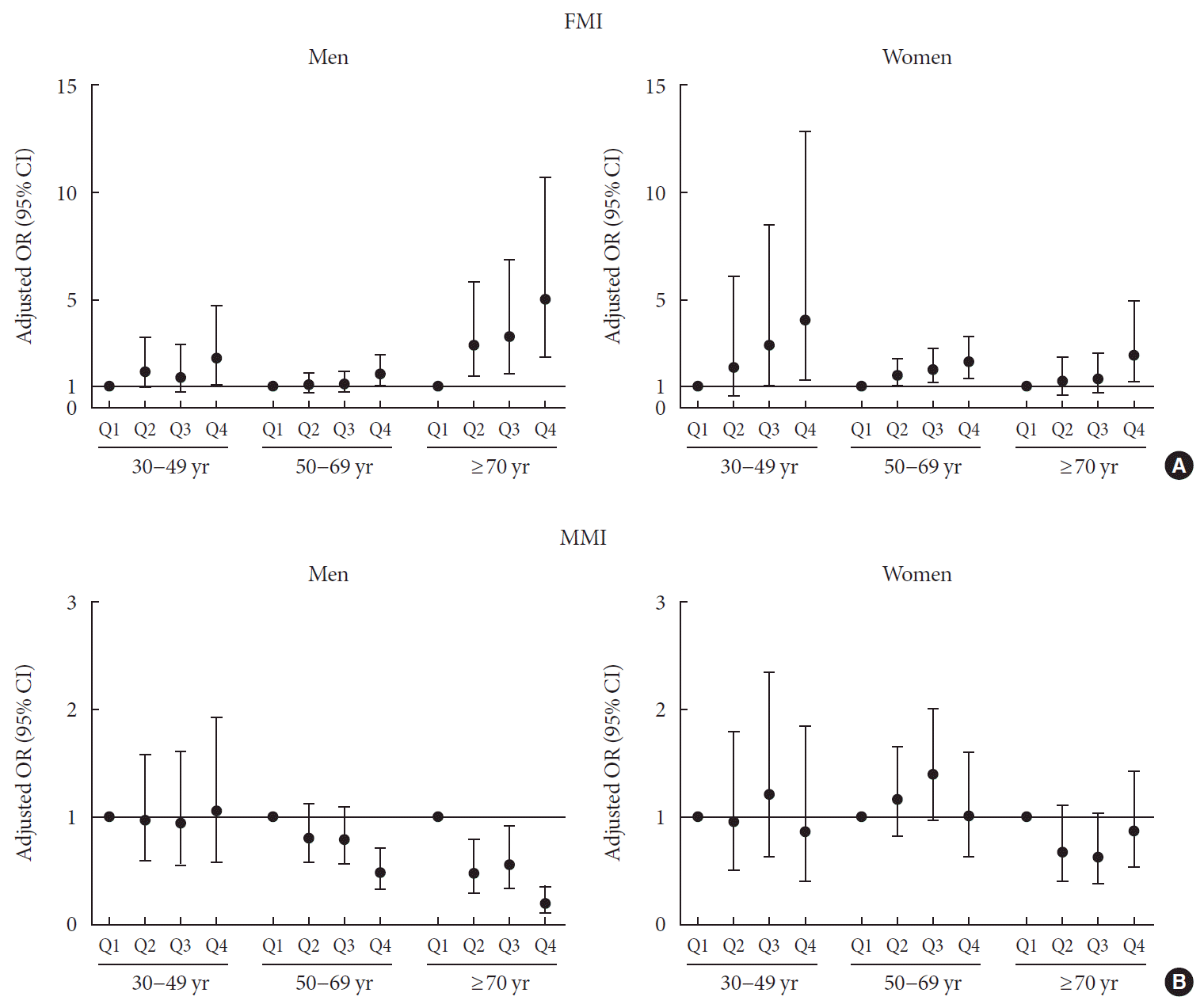
| FMI, m2 | 0.001a | 0.208 | <0.001a | 0.202 | <0.001a | 0.049a | ||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Q2 | 2.156 (1.135–4.099) | 0.019 | 1.436 (0.738–2.797) | 0.286 | 1.314 (0.911–1.895) | 0.144 | 0.987 (0.655–1.489) | 0.951 | 3.27 (1.693–6.318) | 0.001 | 2.216 (1.098–4.47) | 0.026 |
| Q3 | 2.086 (1.069–4.07) | 0.031 | 1.298 (0.643–2.623) | 0.466 | 1.52 (1.059–2.181) | 0.023 | 1.007 (0.651–1.56) | 0.973 | 3.876 (1.972–7.62) | <0.001 | 2.297 (1.062–4.965) | 0.035 |
| Q4 | 3.358 (1.818–6.206) | 0.001 | 1.993 (0.978–4.062) | 0.058 | 2.409 (1.721–3.372) | <0.001 | 1.394 (0.863–2.251) | 0.174 | 6.302 (3.234–12.282) | <0.001 | 3.116 (1.405–6.914) | 0.005 |
| MMI, m2 | 0.09 | <0.001a | 0.12 | <0.001a | 0.001a | |||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Q2 | 0.739 (0.46–1.187) | 0.21 | 0.695 (0.505–0.956) | 0.026 | 0.849 (0.607–1.187) | 0.337 | 0.468 (0.286–0.765) | 0.003 | 0.521 (0.312–0.868) | 0.012 | ||
| Q3 | 0.649 (0.392–1.074) | 0.093 | 0.65 (0.481–0.878) | 0.005 | 0.938 (0.665–1.323) | 0.713 | 0.497 (0.303–0.815) | 0.006 | 0.665 (0.393–1.126) | 0.128 | ||
| Q4 | 0.515 (0.3–0.882) | 0.016 | 0.346 (0.241–0.497) < | 0.001 | 0.621 (0.411–0.937) | 0.024 | 0.162 (0.089–0.294) | <0.001 | 0.295 (0.157–0.554) | 0.001 | ||
| SBP, mm Hg | 1.027 (1.017–1.038) < | 0.001a | 1.007 (0.98–1.034) | 0.63 | 1.01 (1.002–1.017) | 0.009a | 1.032 (1.02–1.044) < | 0.001a | 1.002 (0.992–1.012) | 0.71 | ||
| DBP, mm Hg | 1.036 (1.022–1.05) < | 0.001a | 1.011 (0.976–1.047) | 0.543 | 0.987 (0.976–0.998) | 0.02a | 0.944 (0.927–0.961) < | 0.001a | 0.978 (0.959–0.997) | 0.024a | 0.968 (0.948–0.989) | 0.003a |
| HDL-C, mg/dL | 0.975 (0.953–0.997) | 0.029a | 0.992 (0.969–1.016) | 0.515 | 0.97 (0.958–0.982) < | 0.001a | 0.983 (0.971–0.995) | 0.005a | 0.97 (0.95–0.99) | 0.003a | 0.983 (0.961–1.005) | 0.122 |
| TG, mg/dL | 1.002 (1.001–1.003) < | 0.001a | 1.001 (1–1.002) | 0.026a | 1.002 (1.001–1.002) | 0.001a | 1.001 (1–1.002) | 0.066 | 1.003 (1.001–1.005) | 0.01 | 1 (0.997–1.002) | 0.983 |
| AST, IU/L | 1.009 (1.002–1.016) | 0.016a | 1.005 (1–1.011) | 0.063 | 1.006 (0.999–1.013) | 0.071 | 1.005 (0.995–1.016) | 0.328 | ||||
| ALT, IU/L | 1.006 (0.997–1.015) | 0.191 | 1.014 (1.007–1.02) < | 0.001a | 1.01 (1.003–1.017) | 0.006a | 1.025 (1.011–1.039) | 0.001a | 1.023 (1.009–1.038) | 0.002a | ||
| Low income | 1.491 (1–2.222) | 0.049a | 1.548 (1.022–2.344) | 0.039a | 1.588 (1.195–2.109) | 0.002a | 1.631 (1.209–2.201) | 0.001a | 0.967 (0.629–1.487) | 0.879 | ||
| Current smoking | 1.114 (0.791–1.568) | 0.535 | 1.076 (0.849–1.363) | 0.545 | 0.981 (0.632–1.522) | 0.931 | ||||||
| Drinking | 0.905 (0.573–1.429) | 0.669 | 0.913 (0.713–1.168) | 0.466 | 0.81 (0.56–1.172) | 0.263 | ||||||
| Regular exercise | 1.167 (0.776–1.754) | 0.458 | 0.899 (0.669–1.207) | 0.477 | 0.859 (0.463–1.592) | 0.628 | ||||||
ORs of diabetes mellitus per one-unit increase in age, BMI, SBP, DBP, HDL-C, TG, AST, ALT or one quartile of FMI/MMI or presence of low income, current smoking, drinking, or regular exercise.
OR, odds ratio; CI, confidence interval; BMI, body mass index; FMI, fat mass index; MMI, muscle mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 3.
| Variable |
30–49 yr |
50–69 yr |
≥70 yr |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | P value | Multivariate | P value | Univariate | P value | Multivariate | P value | Univariate | P value | Multivariate | P value | |
| Age, yr | 1.12 (1.072–1.171) | <0.001a | 1.097 (1.044–1.152) | 0.001a | 1.086 (1.066–1.107) | <0.001a | 1.078 (1.054–1.102) | <0.001a | 0.98 (0.946–1.016) | 0.272 | ||
| BMI, kg/m2 | 1.234 (1.177–1.295) | <0.001a | 1.113 (1.03–1.203) | 0.007a | 1.142 (1.1–1.185) | <0.001a | 1.067 (1.011–1.127) | 0.019 | 1.143 (1.09–1.198) | <0.001a | 1.059 (0.991–1.133) | 0.091 |
| FMI, m2 | <0.001a | 0.102 | <0.001a | 0.036a | <0.001a | 0.045a | ||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Q2 | 3.053 (0.91–10.248) | 0.071 | 1.498 (0.433–5.185) | 0.523 | 1.827 (1.225–2.725) | 0.003 | 1.41 (0.927–2.146) | 0.108 | 1.57 (0.884–2.79) | 0.124 | 1.248 (0.651–2.396) | 0.504 |
| Q3 | 6.418 (2.158–19.09) | 0.001 | 2.881 (0.917–9.049) | 0.07 | 2.53 (1.71–3.744) | <0.001 | 1.631 (1.064–2.501) | 0.025 | 1.905 (1.097–3.311) | 0.022 | 1.256 (0.649–2.431) | 0.499 |
| Q4 | 14.441 (5.109–40.819) | <0.001 | 3.997 (1.184–13.493) | 0.026 | 3.387 (2.324–4.936) | <0.001 | 1.891 (1.229–2.908) | 0.004 | 3.939 (2.324–6.676) | <0.001 | 2.275 (1.103–4.69) | 0.026 |
| MMI, m2 | 0.002a | 0.956 | 0.003a | 0.16 | 0.004a | 0.119 | ||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Q2 | 0.62 (0.344–1.119) | 0.112 | 0.95 (0.507–1.78) | 0.872 | 0.849 (0.617–1.169) | 0.316 | 1.209 (0.836–1.749) | 0.312 | 0.574 (0.35–0.942) | 0.028 | 0.688 (0.407–1.162) | 0.162 |
| Q3 | 0.526 (0.29–0.955) | 0.035 | 1.163 (0.585–2.31) | 0.666 | 0.92 (0.668–1.266) | 0.607 | 1.515 (1.036–2.217) | 0.032 | 0.467 (0.289–0.755) | 0.002 | 0.651 (0.386–1.098) | 0.108 |
| Q4 | 0.259 (0.129–0.521) | 0.001 | 1.048 (0.501–2.192) | 0.902 | 0.497 (0.339–0.731) | 0.001 | 1.146 (0.712–1.843) | 0.575 | 0.517 (0.338–0.793) | 0.003 | 1.09 (0.646–1.838) | 0.747 |
| SBP, mm Hg | 1.035 (1.024–1.046) | <0.001a | 1.024 (0.995–1.053) | 0.105 | 1.021 (1.002–1.005) | <0.001a | 1.012 (1.005–1.019) | 0.001a | 1.011 (1.003–1.02) | 0.009a | 1.011 (1.001–1.021) | 0.031a |
| DBP, mm Hg | 1.043 (1.027–1.057) | <0.001a | 0.973 (0.933–1.015) | 0.2 | 0.997 (0.987–1.008) | 0.615 | 0.991 (0.973–1.01) | 0.347 | ||||
| HDL-C, mg/dL | 0.956 (0.935–0.976) | <0.001a | 0.992 (0.969– 1.016) | 0.52 | 0.968 (0.955–0.981) | <0.001a | 0.986 (0.971– 1.002) | 0.082 | 0.987 (0.971–1.003) | 0.123 | ||
| TG, mg/dL | 1.006 (1.004–1.009) | <0.001a | 1.003 (1.001–1.006) | 0.006a | 1.004 (1.002–1.005) | <0.001a | 1.002 (1–1.003) | 0.015a | 1.003 (1.001–1.005) | 0.001a | 1.002 (1–1.004) | 0.023a |
| AST, IU/L | 1.056 (1.032–1.081) | <0.001a | 1.019 (0.954–1.089) | 0.579 | 1.015 (1.006–1.024) | 0.002a | 0.974 (0.946–1.003) | 0.075 | 1.018 (1–1.037) | 0.046a | 0.958 (0.924–0.992) | 0.017a |
| ALT, IU/L | 1.031 (1.176–3.072) | <0.001a | 1.01 (0.979– 1.042) | 0.538 | 1.019 (1.01–1.029) | <0.001a | 1.029 (1.009–1.05) | 0.005a | 1.041 (1.025–1.057) | <0.001a | 1.061 (1.03–1.092) | <0.001a |
| Low income | 1.901 (0.097–1.379) | 0.009a | 1.604 (0.962– 2.673) | 0.07 | 1.006 (0.753–1.345) | 0.965 | 0.784 (0.549–1.121) | 0.183 | ||||
| Current smoking | 0.366 (0.097–1.379) | 0.137 | 0.465 (0.229–0.946) | 0.035a | 0.492 (0.237.1.02) | 0.057 | 1.058 (0.534–2.097) | 0.87 | ||||
| Drinking | 0.954 (0.592–1.537) | 0.847 | 0.683 (0.51–0.915) | 0.011a | 0.847 (0.612–1.171) | 0.313 | 0.991 (0.619–1.586) | 0.969 | ||||
| Regular exercise | 0.788 (0.39–1.595) | 0.508 | 0.809 (0.538–1.217) | 0.309 | 1.088 (0.589–2.011) | 0.787 | ||||||
ORs of diabetes mellitus per one-unit increase in age, BMI, SBP, DBP, HDL-C, TG, AST, ALT or one quartile of FMI/MMI or presence of low income, current smoking, drinking, or regular exercise.
OR, odds ratio; CI, confidence interval; BMI, body mass index; FMI, fat mass index; MMI, muscle mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download