Abstract
Background
Methods
Results
Notes
References
Fig. 1
Odds ratio for (A) increased serum immunoglobulin E (IgE) and (B) atopy according to the metabolic status and obesity. The study population was stratified by sex and menopausal status. MHNO, metabolically healthy nonobese; MUNO, metabolically unhealthy nonobese; MHO, metabolically healthy obese; MUO, metabolically unhealthy obese.
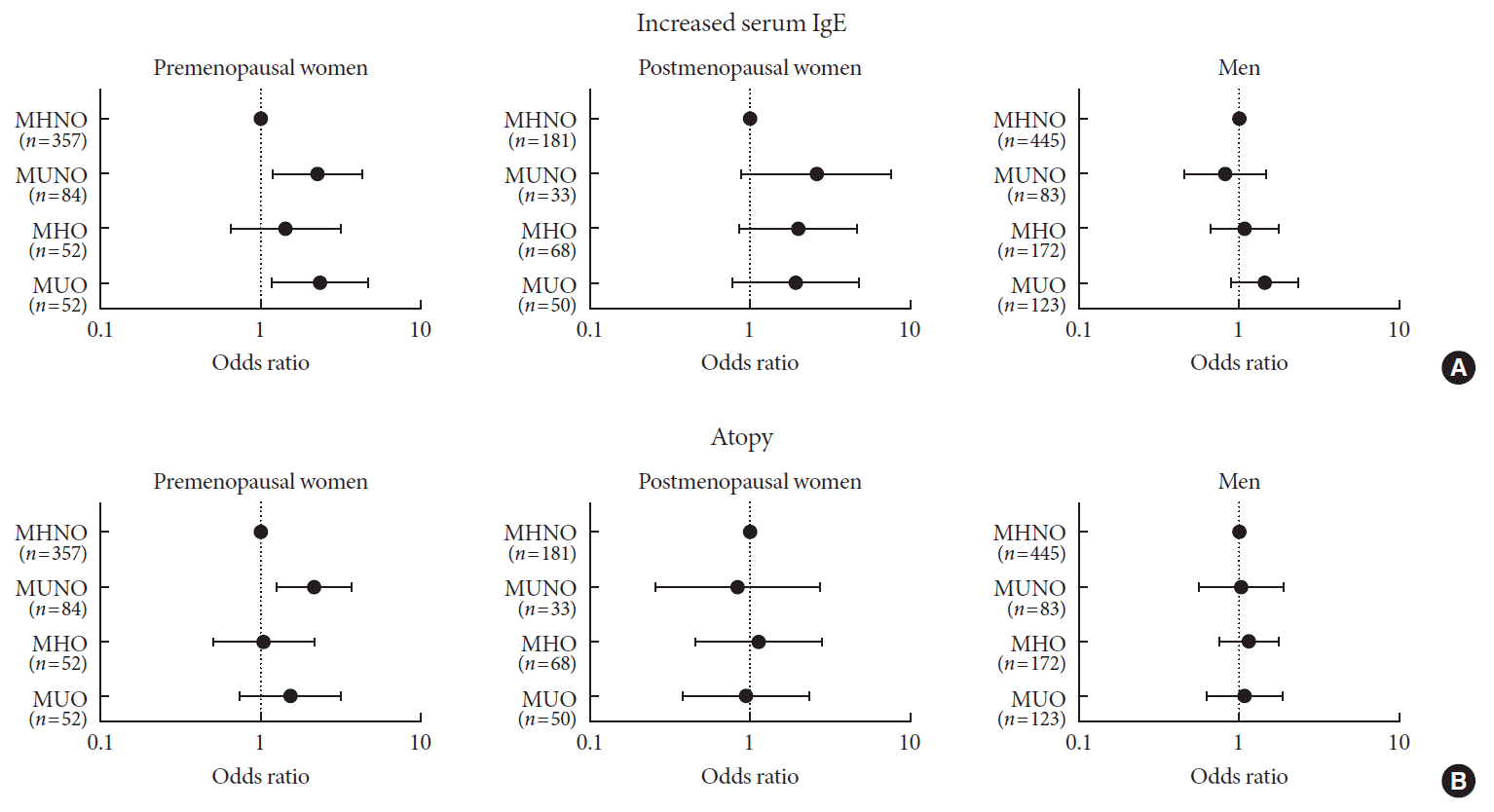
Table 1.
| Characteristic | Premenopausal women (n=545) | Postmenopausal women (n=332) | Men (n=823) | P value |
|---|---|---|---|---|
| Age, yr | 35.3±0.4a,b | 61.2±0.8b,c | 42.5±0.6a,c | <0.001 |
| BMI, kg/m2 | 22.6±0.2a,b | 24.5±0.3c | 24±0.1c | <0.001 |
| Waist circumference, cm | 74.8±0.5a,b | 82.3±0.7c | 83.6±0.46c | <0.001 |
| Ever smoker, % | 12.8 (1.9)b | 8.3 (2.4)b | 76.1 (1.8)a,c | <0.001 |
| Heavy drinker, % | 3.4 (1.1)b | 0.9 (0.6)b | 16.4 (1.5)a,c | <0.001 |
| Physically active subjects, % | 18.3 (1.8)b | 23.8 (3.4) | 29.8 (1.9)c | <0.001 |
| 25-hydroxyvitamin D, ng/mL | 16±0.4a,b | 18.4±0.6c | 19.4±0.46c | <0.001 |
| Insulin resistance | ||||
| HOMA-IR | 2.19 (2.09–2.29) | 2.26 (2.14–2.39) | 2.19 (2.11–2.28) | 0.559 |
| Insulin, µIU/mL | 9.91 (9.52–10.31) | 9.7 (9.26–10.17) | 9.58 (9.27–9.9) | 0.417 |
| Allergen sensitization | ||||
| Total IgE, kU/L | 59.8 (51.9–69)b | 62.3 (50.1–77.5)b | 128.4 (113.5–145.3)a,c | <0.001 |
| Dust mite-specific IgE, kU/L | 0.177 (0.14–0.222)a,b | 0.09 (0.066–0.121)b,c | 0.36 (0.296–0.438)a,c | <0.001 |
| Cockroach-specific IgE, kU/L | 0.067 (0.057–0.078)b | 0.064 (0.053–0.078)b | 0.137 (0.12–0.157)a,c | <0.001 |
| Dog-specific IgE, kU/L | 0.026 (0.022–0.029)b | 0.022 (0.019–0.025)b | 0.039 (0.034–0.045)a,c | <0.001 |
Table 2.
| BMI, kg/m2 | HOMA-IR | Total IgE, kU/L | P value | Dust mite-specific IgE, kU/L | P value | Cockroach-specific IgE, kU/L | P value | Dog-specific IgE, kU/L | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal women | 0.015a | 0.101 | 0.035a | 0.042a | ||||||
| Underweight, <18.5 (n=50) | 2.00 (1.90–2.11) | 39.6 (26.9–58.3) | 0.066 (0.032–0.134) | 0.043 (0.032–0.057) | 0.017 (0.012–0.023) | |||||
| Healthy weight, 18.5–22.9 (n=306) | 2.35 (2.11–2.61) | 55.5 (46.2–66.8) | 0.167 (0.121–0.231) | 0.063 (0.052–0.077) | 0.024 (0.020–0.029) | |||||
| Overweight, 23.0–24.9 (n=85) | 1.83 (1.65–2.04) | 65.7 (46.7–92.5) | 0.281 (0.136–0.581) | 0.083 (0.056–0.125) | 0.028 (0.022–0.036) | |||||
| Obesity, ≥25.0 (n=104) | 2.77 (2.52–3.04) | 79.1 (57.4–108.9) | 0.221 (0.118–0.415) | 0.079 (0.056–0.111) | 0.033 (0.023–0.046) | |||||
| Postmenopausal women | 0.333 | 0.581 | 0.598 | 0.536 | ||||||
| Underweight, <18.5 (n=3) | 1.92 (1.77–2.08) | 77.5 (20.0–300.7) | 0.047 (0.017–0.134) | 0.062 (0.015–0.257) | 0.015 (0.008–0.030) | |||||
| Healthy weight, 18.5–22.9, (n=119) | 2.28 (2.11–2.46) | 54.2 (37.9–77.5) | 0.077 (0.052–0.116) | 0.065 (0.045–0.093) | 0.021 (0.016–0.028) | |||||
| Overweight, 23.0–24.9 (n=92) | 1.34 (1.29–1.39) | 50.5 (33.8–75.5) | 0.097 (0.059–0.160) | 0.069 (0.050–0.096) | 0.020 (0.016–0.025) | |||||
| Obesity, ≥25.0 (n=118) | 2.62 (2.40–2.85) | 70.2 (49.6–99.4) | 0.093 (0.053–0.162) | 0.057 (0.042–0.077) | 0.024 (0.019–0.030) | |||||
| Men | 0.106 | 0.594 | 0.015a | 0.668 | ||||||
| Underweight, 18.5 (n=23) | 1.83 (1.74–1.92) | 56.1 (24.5–128.4) | 0.096 (0.027–0.339) | 0.072 (0.038–0.138) | 0.023 (0.015–0.035) | |||||
| Healthy weight, 18.5–22.9 (n=303) | 2.20 (2.09–2.32) | 128.1 (105.1–156.2) | 0.386 (0.277–0.537) | 0.124 (0.099–0.155) | 0.040 (0.032–0.051) | |||||
| Overweight, 23.0–24.9 (n=202) | 1.73 (1.50–1.99) | 126.7 (101.2–158.7) | 0.350 (0.229–0.536) | 0.120 (0.091–0.159) | 0.037 (0.028–0.047) | |||||
| Obesity, ≥25.0 (n=295) | 2.71 (2.54–2.89) | 147.2 (120.8–179.3) | 0.363 (0.266–0.495) | 0.177 (0.139–0.224) | 0.040 (0.033–0.049) | |||||
Values are presented as geometric mean (95% confidence interval). Values of total and specific IgE levels were adjusted for age, vitamin D, smoking status, drinking status, and physical activity.
BMI, body mass index; IgE, immunoglobulin E; HOMA-IR, homeostatic model assessment of insulin resistance.
Table 3.
| Variable | Total IgE, kU/L | P value | Dust mite-specific IgE, kU/L | P value | Cockroach-specific IgE, kU/L | P value | Dog-specific IgE, kU/L | P value |
|---|---|---|---|---|---|---|---|---|
| Premenopausal women | 0.009a | 0.003a | 0.003a | 0.025a | ||||
| HOMA-IR Q1 | 48.1 (37.5–61.9) | 0.094 (0.064–0.139) | 0.054 (0.043–0.066) | 0.018 (0.015–0.022) | ||||
| HOMA-IR Q2 | 55.8 (43.6–71.3) | 0.203 (0.122–0.336) | 0.060 (0.047–0.078) | 0.032 (0.024–0.042) | ||||
| HOMA-IR Q3 | 55.8 (43.0–72.5) | 0.137 (0.082–0.229) | 0.066 (0.049–0.088) | 0.024 (0.019–0.030) | ||||
| HOMA-IR Q4 | 81.7 (62.2–107.3) | 0.343 (0.192–0.615) | 0.092 (0.067–0.125) | 0.031 (0.023–0.041) | ||||
| Postmenopausal women | 0.077 | 0.304 | 0.992 | 0.336 | ||||
| HOMA-IR Q1 | 45.2 (30.1–67.8) | 0.067 (0.036–0.125) | 0.063 (0.041–0.096) | 0.020 (0.014–0.027) | ||||
| HOMA-IR Q2 | 66.5 (43.5–101.7) | 0.086 (0.048–0.153) | 0.065 (0.045–0.094) | 0.021 (0.016–0.027) | ||||
| HOMA-IR Q3 | 49.5 (31.3–78.5) | 0.105 (0.048–0.231) | 0.059 (0.043–0.082) | 0.023 (0.016–0.031) | ||||
| HOMA-IR Q4 | 82.4 (56.5–120.1) | 0.097 (0.062–0.152) | 0.064 (0.042–0.098) | 0.024 (0.018–0.031) | ||||
| Men | 0.178 | 0.311 | 0.250 | 0.371 | ||||
| HOMA-IR Q1 | 130.3 (101.7–167.0) | 0.325 (0.209–0.506) | 0.133 (0.101–0.175) | 0.037 (0.028–0.049) | ||||
| HOMA-IR Q2 | 103.0 (80.0–132.6) | 0.254 (0.172–0.376) | 0.118 (0.090–0.154) | 0.035 (0.027–0.046) | ||||
| HOMA-IR Q3 | 145.9 (116.5–182.7) | 0.520 (0.341–0.793) | 0.147 (0.109–0.198) | 0.042 (0.032–0.054) | ||||
| HOMA-IR Q4 | 148.6 (117.3–188.2) | 0.358 (0.242–0.529) | 0.155 (0.123–0.195) | 0.042 (0.032–0.055) |
Values are presented as geometric mean (95% confidence interval). Subjects were divided into four categories (Q1 to Q4), ranging from the lowest quartile group to the highest quartile group. Values of total and specific IgE levels were adjusted for age, vitamin D, smoking status, drinking status, and exercise.
HOMA-IR, homeostatic model assessment of insulin resistance; IgE, immunoglobulin E.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download