Abstract
Objective
This study aimed to investigate the prognosis of patients with intermediate-risk cervical cancer and to evaluate the necessity of adjuvant therapy.
Methods
We conducted a retrospective chart review of patients with stage IB–II cervical cancer who underwent type III radical hysterectomy with pelvic lymphadenectomy between 2008 and 2017. In our institution, radical hysterectomy is performed as an open surgery and not as a minimally invasive surgery, and adjuvant therapy is not administered to patients with intermediate-risk cervical cancer. The intermediate-risk group included patients with 2 or more of the following factors: tumor size >4 cm, stromal invasion >1/2, and lymphovascular stromal invasion. Intermediate-risk patients with squamous cell carcinoma were included in the I-SCC group, whereas those with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma were included in the I-Adeno group.
Results
There were 34 and 18 patients in the I-SCC and I-Adeno groups, respectively. The 5-year recurrence-free survival (RFS) and overall survival rates in the I-SCC group were 90.5% (95% confidence interval [CI], 85.3–95.7%) and 100% (95% CI, 100%), respectively, whereas those in the I-Adeno group were 54.9% (95% CI, 42.0–67.9%) and 76.1% (95% CI, 63.7–88.4%), respectively. Multivariate analysis revealed that endocervical adenocarcinoma, usual type, or adenosquamous carcinoma, and tumor size >4 cm had worse RFS.
Early stage cervical cancer is treated with radical hysterectomy with pelvic lymphadenectomy, and adjuvant therapy is recommended depending on the presence of risk factors for recurrence. Adjuvant radiotherapy is indicated for patients in the intermediate-risk group, which is characterized by the negative pelvic lymph nodes, parametrial invasion, and a combination of specific values of tumor size, lymphovascular space invasion (LVSI), and depth of stromal invasion. The evidence for the efficacy of adjuvant radiotherapy originates from a randomized study, the Gynecologic Oncology Group (GOG) 92 [1,2]. The GOG 92 trial showed that the adjuvant radiotherapy arm had improved progression-free survival compared to the surgery-only arm.
It has been pointed out that the GOG 92 trial had some limitations [3]. The GOG 92 trial was conducted more than 20 years ago, and the diagnostic and treatment techniques were different from those used in the present. Tumor size was estimated visually, and recurrence in the surgery-only arm was poor compared to current expectations. Furthermore, the radiotherapy arm tended to have prolonged overall survival (OS) but did not show significant differences.
We do not perform adjuvant therapy for patients with intermediate-risk cervical cancer at our institution. The prognoses of patients show the baseline risks of recurrence and death, and the data are based on current diagnostic and therapeutic methods. To the best of our knowledge, prognostic data for patients in the intermediate-risk group who had no adjuvant therapy are few in recent years [3,4]. We believe that our prognostic data are valuable for reviewing the GOG 92 trial. The present study aimed to investigate the prognosis of early cervical cancer patients with intermediate risk based on histological types. Moreover, we aimed to evaluate the necessity of adjuvant therapy.
We performed type III radical hysterectomy with pelvic lymphadenectomy for stage IB–II cervical cancer classified according to the International Federation of Gynecology and Obstetrics (FIGO) 2008 system. Radical hysterectomy is performed as an open surgery and not as a minimally invasive surgery in our institution. Patients with positive lymph node metastasis, positive parametrial invasion, or positive surgical margin received adjuvant concurrent chemoradiotherapy (CCRT), while the other patients did not.
We collected cases of patients with stage IB–II (FIGO 2008) cervical cancer who underwent type III radical hysterectomy with pelvic lymphadenectomy between January 2008 and December 2017. Patients with positive lymph node metastasis, positive parametrial invasion, positive surgical margins, and ovarian metastasis were excluded. The histological types included limited squamous cell carcinoma (SCC), endocervical adenocarcinoma, usual type (AC), and adenosquamous carcinoma (AS). Patients’ prognoses, and clinical and tumor pathological information were obtained from medical records, including age, body mass index (BMI), histological type, tumor size, stromal invasion, lymphovascular stromal invasion (LVSI), and vaginal invasion.
The low-risk group included patients with less than 2 and intermediate-risk group included patients with 2 or more of the following factors: tumor size >4 cm, stromal invasion >1/2, and LVSI. In the intermediate-risk group, the I-SCC group included patients with SCC, while the I-Adeno group included patients with AC or AS. Similarly, in the low-risk group, the L-SCC group comprised patients with SCC, while the L-Adeno group comprised patients with AC or AS.
Categorical variables were compared using the χ2 test, whereas continuous variables were compared using the Mann-Whitney U test. For survival analysis, recurrence-free survival (RFS) was defined as the period from the date of operation to the date of first recurrence or date of death due to any cause. OS was defined as the period from the date of operation to the date of death of any cause. Survival curves were constructed using the Kaplan-Meier method, and a univariate log-rank test was used to assess the statistical significance. Multivariate analyses for RFS were performed using the Cox proportional hazard model. All statistical analyses were performed using JMP® 15 (SAS Institute Inc., Cary, NC, USA).
Overall, 213 patients with stage IB–II cervical cancer who underwent type III radical hysterectomy with pelvic lymphadenectomy were identified during the study period. Of the 213 patients, 128 met the inclusion criteria and 85 were excluded, as they were classified into the high-recurrence group or as a rare histologic type (other than SCC, AC, or AS). A total of 52 patients were included in the intermediate-risk group, with 34 patients in the I-SCC group and 18 in the I-Adeno group. There were 76 patients included in the low-risk group, with 38 in the L-SCC group, and 38 in the L-Adeno group (Fig. 1). The characteristics of the I-SCC and I-Adeno groups are summarized in Table 1. The median age was 45 years (range, 25–63 years) and median BMI was 20.8 (range, 16.8–29.8) in the I-SCC group, while in the I-Adeno group, the median age was 48 years (range, 39–66 years) and the median BMI was 20.6 (range, 17.2–34.2). Ten patients (29.4%) had a tumor size >4 cm, 33 patients (97.1%) had stromal invasion >1/2, 33 patients (97.1%) had positive LVSI, and 10 patients (29.4%) had positive vaginal invasion in the I-SCC group. Five patients (27.8%) had tumor size >4 cm, 17 patients (94.4%) had stromal invasion >1/2, 16 patients (88.9%) had positive LVSI, and 6 patients (33.3%) had positive vaginal invasion in the I-Adeno group. No significant differences in patient characteristics were observed between the I-SCC and I-Adeno groups. The characteristics of patients within the L-SCC and L-Adeno groups are summarized in Supplementary Table 1. The L-Adeno group had a higher proportion of patients with positive vaginal invasion than the L-SCC group (P =0.04).
The median follow-up period was 59 months (range, 2–131 months). During the follow-up period, 3 (8.8%) patients had recurrence and no (0.0%) patients died in the I-SCC group, while 7 (38.9%) patients had recurrence and 3 (17.6%) patients died in the I-Adeno group. Kaplan-Meier estimates of RFS and OS of the I-SCC and I-Adeno groups are presented in Fig. 2. The 5-year RFS and 5-year OS rates of the I-SCC group were 90.5% (95% confidence interval [CI], 85.3–95.7%) and 100% (95% CI, 100%), respectively.
On the contrary, the 5-year RFS and 5-year OS rates of the I-Adeno group were 54.9% (95% CI, 42.067.9%) and 76.1% (95% CI, 63.7–88.4%), respectively. Patients in the I-SCC group had significantly higher RFS and OS than those in the I-Adeno group (P <0.01, P =0.02). In the low-risk group, no patients had recurrence or died in the L-SCC group, and only 1 patient had recurrence and no patients died in the L-Adeno group. There were no significant differences in RFS and OS between the I-SCC and L-SCC groups, while patients in the I-Adeno group had significantly lower RFS and OS than patients in the L-Adeno group (P <0.01, P <0.01). Kaplan-Meier estimates of RFS and OS of the I-Adeno and L-Adeno groups are presented in Fig. 3. Multivariate analysis with histological type, tumor size, LVSI, and vaginal invasion revealed that histological type and tumor size had a significant effect on RFS (Table 2). Patients with AC or AS had low rates of RFS (hazard ratio [HR], 5.66; 95% CI, 1.38–23.22); patients with a tumor size >4 cm had low rates of RFS (HR, 9.68; 95% CI, 2.57–36.42). As all patients who had recurrence had stromal invasion >1/2 in our study, the HR could not be calculated by the depth of stromal invasion. Thus, we did not include the depth of stromal invasion in the multivariate analysis.
The outcomes of recurrence of cases in the I-SCC and I-Adeno groups are shown in Table 3. In the I-SCC group, 3 patients had recurrent disease; 1 involved an intrapelvic lymph node, 1 involved a para-aortic lymph node, and 1 had vaginal stump recurrence and involved a para-aortic lymph node. These patients received salvage radiotherapy upon recurrence. In the I-Adeno group, 7 patients had recurrent disease; 5 had intrapelvic recurrence and 2 had extrapelvic recurrence, while 3 patients died.
Our study showed the baseline recurrence risk of cervical cancer in the intermediate-risk group based on current diagnostic and therapeutic methods. Regarding SCC, the I-SCC group showed good prognosis without adjuvant therapy and had no significant differences in prognosis compared to the L-SCC group. On the contrary, the I-Adeno group showed a high recurrence rate without adjuvant therapy and had poor prognosis compared to the L-Adeno and I-SCC groups.
The criteria for categorizing patients in the intermediate-risk group of cervical cancer are different in each guideline or study protocol. The National Comprehensive Cancer Network guidelines [5] introduced the Sedlis criteria for intermediate-risk factors, which were adopted in the GOG 92 trial [1,2]. The guidelines defined the intermediate-risk group as including patients with at least 2 of the following risk factors: 1) greater than one-third stromal invasion, 2) capillary lymphatic space involvement, and 3) cervical tumor diameters of >4 cm. The Japan Society of Gynecologic Oncology (JSGO) guidelines define the intermediate-risk group as including patients with any of the following risk factors: 1) large tumor size, 2) deep stromal invasion, and 3) LVSI [6]. The Korean Gynecologic Oncology Group (KGOG) advocates a “4-factor model” that defines the intermediate-risk group as including patients with at least 2 of the following risk factors: 1) AC or AS, 2) tumor size ≥3 cm, 3) stromal invasion ≥outer 1/3, and 4) LVSI [7]. We adopted the modified Sedlis criteria to simplify the classification at our institution. The same patients would have been included in the present study even if we had adopted the original Sedlis criteria.
In the present study, the recurrence rate was 8.8% (3/34) in the I-SCC group, and all 3 patients were salvaged. The recurrence rate was lower than that of the surgery-only arm of the GOG 92 trial (27.8%, 32/115). Some of the factors for this difference may be the transition in preoperative diagnosis and the improvement of surgical techniques. Cibula et al. [3] showed the prognosis of the intermediate-risk group without adjuvant therapy. In the study, the intermediate-risk group was defined as cases that met the following criteria: LVSI and deep stromal invasion, LVSI and tumor size ≥2 cm, or tumor size ≥4 cm. The recurrence and mortality rates were 6.3% (8/127) and 7.1% (9/127), respectively, and the 5-year RFS and 5-year OS rates were 94.5% and 95.7%, respectively. These results are similar to our findings, besides the small difference in inclusion criteria. Furthermore, the prognosis of the no adjuvant therapy group was not inferior to that of the adjuvant radiotherapy group. The necessity of adjuvant therapy should be considered based on the baseline recurrence risk, efficacy of recurrence prevention, and complications arising in the treatment. Complications associated with radiotherapy included nausea, diarrhea, anemia, leg edema, ileus, lymphedema, and bone fracture, among others. The GOG 92 trial reported that grade 3 and 4 adverse events were seen in 7% of the radiotherapy arm, and other studies reported that adjuvant radiotherapy increased the occurrence of adverse events [1,2,8,9]. Regarding SCC, the benefit of adjuvant therapy for patients in the intermediate-risk group might be small, as the baseline recurrence and mortality rates were low. Adjuvant therapy might be omitted because it has little benefits and a considerable number of risks.
Lai et al. [10] reported no significant differences in RFS between adenocarcinoma and adenosquamous carcinoma, and Baek et al. [11] reported no differences in patterns of recurrence and time to recurrence. We analyzed AC, and AS together, as in the GOG 92 trial. The prognosis of the I-Adeno group was inferior to that of the I-SCC or L-Adeno groups, and multivariate analysis showed that AC or AS had worse RFS in the present study. Other studies also reported that the outcomes of cervical adenocarcinoma were inferior to that of SCC [12–14]. The “4-factor model,” suggested by the KGOG study as the new criteria for the intermediate-risk group, is unique compared to the Sedlis or JSGO criteria in that it contains the criterion of histological type [5–7]. The histological type of the tumor was a significant prognostic factor in the present study. Our study showed that the 5-year RFS rate of the I-Adeno group was as poor as 54.9%. Because the baseline recurrence risk of the I-Adeno group was high, adjuvant therapy should be considered in this case. Although the standard adjuvant therapy is radiotherapy based on the GOG 92 trial, studies have reported the possibility that chemotherapy is as effective as adjuvant therapy for intermediate-risk cervical cancer [15–17]. Moreover, the efficacy of CCRT is being studied by GOG 263, which is an ongoing randomized controlled trial comparing CCRT and radiotherapy as adjuvant therapy in patients with intermediate-risk cervical cancer. The results of a subgroup analysis of GOG 263 for adenocarcinoma are noteworthy, and we look forward to future research on adjuvant therapy for cervical adenocarcinoma.
The limitations of our study were the lack of an adjuvant therapy group and the small sample size. It is better to compare the prognosis between the no-adjuvant and adjuvant therapy groups when we evaluate the effectiveness of adjuvant therapy. To be precise, the benefit of adjuvant therapy was unclear in our study, but we consider that the low baseline recurrence risk in the I-SCC group was one of the factors that could lead to omitting adjuvant therapy. Hence, more large-scale cohort studies that contain an adjuvant therapy group are warranted in the future to validate the findings of our study.
In conclusion, regarding SCC, patients with intermediate-risk cervical cancer have good prognosis without adjuvant therapy; therefore, adjuvant therapy may be omitted. On the contrary, regarding adenocarcinoma, patients with intermediate- risk cervical cancer have poor prognosis without adjuvant therapy; adjuvant therapy should therefore be considered in the treatment. However, further large-scale studies are necessary to validate the findings of the present study.
Supplementary materials
Supplementary materials associated with this article can be found online at https://doi.org/10.5468/ogs.20243
Notes
References
1. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999; 73:177–83.


2. Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006; 65:169–76.


3. Cibula D, Abu-Rustum NR, Fischerova D, Pather S, Lavigne K, Slama J, et al. Surgical treatment of “intermediate risk” lymph node negative cervical cancer patients without adjuvant radiotherapy - A retrospective cohort study and review of the literature. Gynecol Oncol. 2018; 151:438–43.


4. Nakamura K, Kitahara Y, Satoh T, Takei Y, Takano M, Nagao S, et al. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC study). World J Surg Oncol. 2016; 14:173.



5. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Cervical cancer version 2. 2020 [Internet]. In : Plymouth Meeting, PA: NCCN; 2020; [cited 2020 Oct 2]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
.
6. Ebina Y, Mikami M, Nagase S, Tabata T, Kaneuchi M, Tashiro H, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019; 24:1–19.


7. Ryu SY, Kim MH, Nam BH, Lee TS, Song ES, Park CY, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer. 2014; 110:278–85.


8. Machida H, Matsuo K, Furusawa A, Kita T, Kitagawa R, Mikami M. Profile of treatment-related complications in women with clinical stage IB-IIB cervical cancer: a nationwide cohort study in Japan. PLoS One. 2019; 14:e0210125.

9. Jung PS, Kim DY, Lee SW, Park JY, Suh DS, Kim JH, et al. Clinical role of adjuvant chemotherapy after radical hysterectomy for FIGO stage IB-IIA cervical cancer: comparison with adjuvant RT/CCRT using inverse-probability-of-treatment weighting. PLoS One. 2015; 10:e0132298.

10. Lai CH, Chou HH, Chang CJ, Wang CC, Hsueh S, Huang YT, et al. Clinical implications of human papillomavirus genotype in cervical adeno-adenosquamous carcinoma. Eur J Cancer. 2013; 49:633–41.


11. Baek MH, Park JY, Kim D, Suh DS, Kim JH, Kim YM, et al. Comparison of adenocarcinoma and adenosquamous carcinoma in patients with early-stage cervical cancer after radical surgery. Gynecol Oncol. 2014; 135:462–7.


12. Huang YT, Wang CC, Tsai CS, Lai CH, Chang TC, Chou HH, et al. Clinical behaviors and outcomes for adenocarcinoma or adenosquamous carcinoma of cervix treated by radical hysterectomy and adjuvant radiotherapy or chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012; 84:420–7.


13. Noh JM, Park W, Kim YS, Kim JY, Kim HJ, Kim J, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13–10). Gynecol Oncol. 2014; 132:618–23.


14. Pan X, Yang W, Wen Z, Li F, Tong L, Tang W. Does adenocarcinoma have a worse prognosis than squamous cell carcinoma in patients with cervical cancer? A real-world study with a propensity score matching analysis. J Gynecol Oncol. 2020; 31:e80.

15. Matsuo K, Shimada M, Yokota H, Satoh T, Katabuchi H, Kodama S, et al. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget. 2017; 8:106866–75.

Fig. 1
Number of patients included for analysis. Thirty-four patients were in the intermediate-risk group with squamous cell carcinoma (I-SCC group) and 18 in the intermediate-risk group with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma (I-Adeno group). Thirty-eight patients were in the low-risk group with squamous cell carcinoma (L-SCC group) and 38 in the low-risk group with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma (L-Adeno group). a)Histologic type other than squamous cell carcinoma,
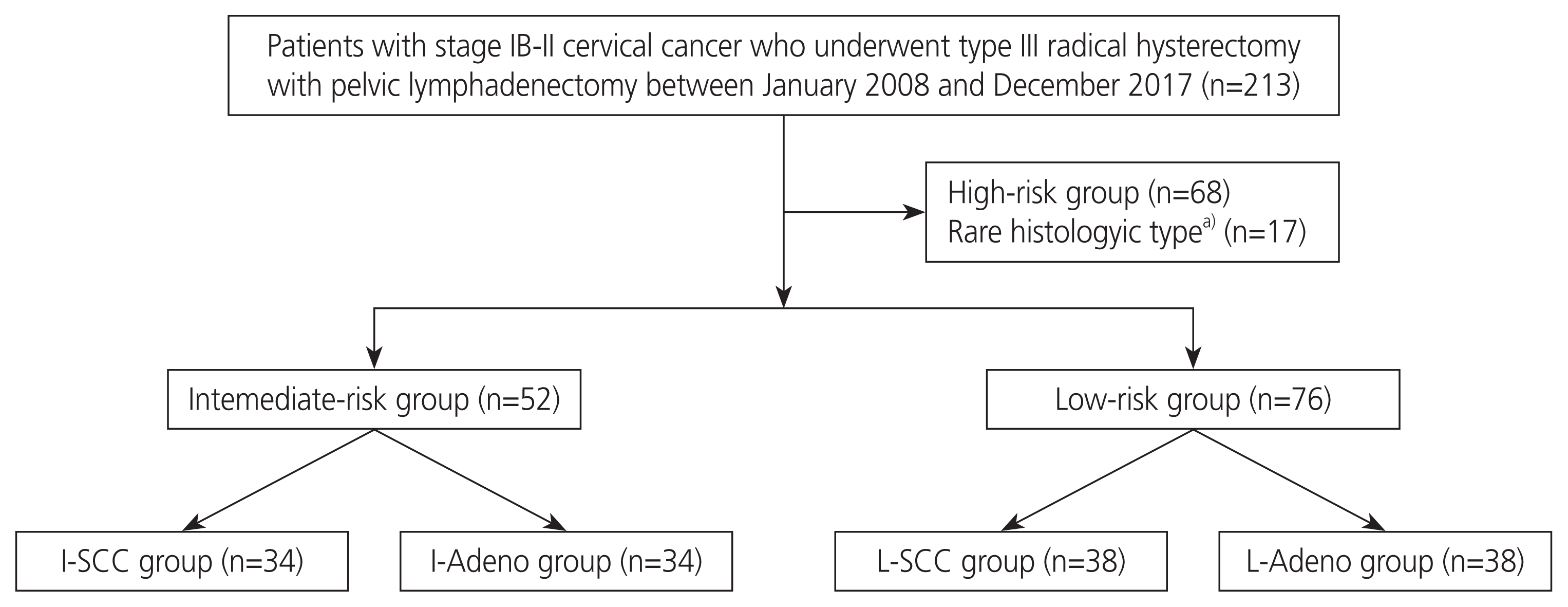
Fig. 2
Kaplan-Meier estimates for recurrence-free survival (RFS) and overall survival (OS) of intermediate-risk group with squamous cell carcinoma (I-SCC group) and intermediate-risk group with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma (I-Adeno group). Patients in the I-SCC group had significantly higher RFS and OS than those in the I-Adeno group (P <0.01, P= 0.02).
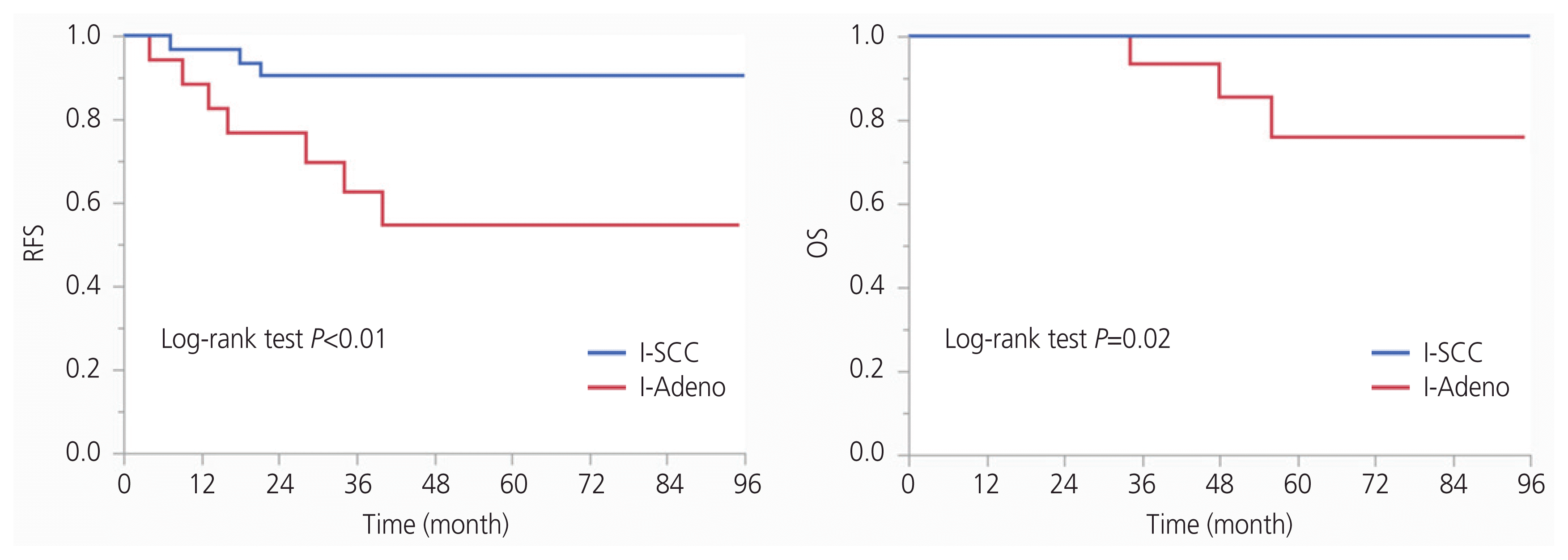
Fig. 3
Kaplan-Meier estimates for recurrence-free survival (RFS) and overall survival (OS) of intermediate-risk group with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma (I-Adeno group) and low-risk group with endocervical adenocarcinoma, usual type, or adenosquamous carcinoma (L-Adeno group). Patients in the I-Adeno group had significantly lower RFS and OS than those in the L-Adeno group (P <0.01, P <0.01).
Table 1
Characteristics of intermediate-risk group
Table 2
Multivariate analysis of clinicopathological factors and recurrence-free survival
Table 3
Outcome of recurrence cases in the intermediate risk group




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download