Abstract
Cavernous sinus (CS) lesion is hard to access by surgical approach. With the development of endovascular technique, neurointerventional therapy is an alternative modality for CS lesions. This endovascular technique has been widely used for the past decade, avoiding the risks associated with surgical treatment. However, complications can still arise from coil embolization. Although immediate complication associate with embolic event or mass effect has been well described, but delayed (>1 year from treatment) nerve palsy after coil embolization is rare. We report two cases of delayed cranial nerve palsy after successful endovascular coil embolization in CS lesion.
The surgical approach of the cavernous sinus (CS) lesion is hard to access. Endovascular treatment of CS lesion is an alternative modality without craniotomy to eliminate the need for additional complication that may arise from microsurgical approach. And the endovascular approach also could reduce the hospital stay with less recovery time. However, complications can still arise from endovascular coil embolization. Thromboembolic events and mass effects are the well-known adverse effects of endovascular treatment. These events are most common during or in the immediate postprocedure period [4]. These complications had been well described, but delayed (>1 year from treatment) nerve palsy after apparently uncomplicated endovascular coil embolization of CS lesion has rarely been reported [2,5]. We describe 2 cases that experienced nerve palsy after coil embolization, and discuss possible mechanisms.
A 61-year-old woman suffered from left hand paresthesia. Angiography demonstrated evidence of incidental unruptured aneurysm (Neck/Height/Width 4.07/12.4/9.2 mm) of the left cavernous internal carotid artery (ICA) (Fig. 1). The aneurysm was embolized with multiple coils (Stryker, Kalamazoo, MI, USA, 360° GDC-18 11×30, GDC-10 SR 10×30, Complex standard 10×30, Complex standard 9×25, GDC-10 2D 9×30, GDC-10 2D 7×25, Complex fill 8×24, total 7) and without any complication, she was discharged (Fig. 1). But 19 months later, she revisited our department due to left side miosis and partial ptosis without anhydrosis (incomplete Horner’s syndrome). Laboratory studies and angiography revealed normal findings (Fig. 1). Although magnetic resonance image (MRI) shows no definite enhancement of the aneurysm and CS, T1 weighted enhanced MRI shows discontinuity of left CS outer wall (Fig. 2). We may assume the coils packed in the aneurysm could migrate to the CS through the hole of the aneurysm. The coils sticking out the aneurysmal wall and inflammation could stimulate the sympathetic plexus that surround the ICA. The patient was treated with intravenous corticosteroid. Despite this therapy, the symptoms are continued. Because the patient did not respond to the corticosteroid treatment, we believed that mechanical compression was the more significant contributing factor for the nerve palsy and may have prevented further recovery.
A 50-year-old woman presented right face paresthesia, exophthalmos and ecchymosis for 7 days. Diplopia was not observed and eye movement was in the normal range. MRI and angiography reveal dural type indirect carotid cavernous fistula (CCF). Preoperative T2-weighted axial MRI shows the signal voiding on right CS and preoperative MR source image shows abnormal signal flow on right CS (Fig. 3). Transvenous embolization was taken with multiple coils (Tornado® Embolization Microcoil™, Cook Incorporated, Bloomington, IN, USA, Tornado 5/2×10, Tornado 4/2×9, total 19). She also was discharged without any focal neurologic deficits. After 38 months, she complained of sudden diplopia. The neurological assessment showed restriction in abduction of the right eye (Abducens nerve impairment). Laboratory tests showed normal limits. Postoperative T2-weighted axial MRI shows some mixed signal intensity lesion around CS and prepontine/superior cerebellopontine cistern, suggesting inflammatory reaction or thrombosis (Fig. 4, C, arrow). But no abnormalities were observed in the hematological examination of the patients. And postoperative MR source image reveals complete obliteration of CCF. After steroid pulse therapy, the diplopia much improved. This cranial nerve palsy may be stabilized with the administration of corticosteroid. Although we could not check follow up MRI, we think the cause of this cranial nerve palsy is inflammatory reaction around the CS.
CS lesion is hard to access by surgical approach. With advance of endovascular technique and device development (microcatheter, microwire and embolization materials), it is becoming the main treatment modality of the CS lesions [3]. Endovascular treatment of CS lesion is an alternative modality without conventional craniotomy and according complications. And it also could reduce hospital stay and recovery time.
Although it has many advantages, complications can still arise from endovascular coil embolization. Categorization of complications after endovascular treatment can be based on the mechanism, etiology and symptom onset timing [10]. The most common and serious neurological complication is thromboembolic stroke, which occurs abruptly and is maximal at onset [4,10]. These events are most common during the procedure or in the immediate postprocedure [4]. These ischemic deficits typically do not improve with time or respond to corticosteroid therapy and may require emergent intervention to reperfusion of the occlude vessels and to recover the nerve injury [10]. Kim et al. report newly developed cranial nerve palsy after transvenous coil embolization. The reason for cranial nerve palsy may be due to progressive thrombosis of the CS, the mass effect due to the packed coils and direct injury by the coil or microwire/microcatheter [6,7]. Progressive cranial nerve palsy typically occurs hours to weeks after the endovascular procedure. Unlike the thromboembolic events, progressive cranial nerve palsy has a slower and more progressive onset. It is likely secondary to evolving mass effect from aneurysm thrombosis with/without perianeurysmal inflammation and edema, or from aneurysmal growth, or from a combination of both processes [8-10]. These nerve palsy may be stabilized or improved with the administration of corticosteroid. These complications described on the above paragraph; occur within a few weeks after coil embolization.
In our cases, however, nerve palsies occurred after 1 year from successful embolization. Delayed (>1 year from treatment) nerve palsy after endovascular coil embolization of CS lesion was rare [2,5]. In generally, after obliteration of the aneurysm by coil embolization, the aneurysmal wall became disorganized from invasion by capillaries and fibroblasts, organization of the thrombotic material, and the initiation of a chronic foreign body reaction in the neighborhood of the coils [1]. The chronic inflammation may cause thickening of the aneurysm wall, making this thicken wall is an important protective mechanism to prevent aneurysm rupture [9]. But in case 1, we hypothesis the coils packed in aneurysm could migrate to the CS through the hole of the aneurysm. The coils sticking out the aneurysmal wall and some inflammation could stimuli the sympathetic trunk that surrounding the ICA. In case 2, we could not explain the inflammation occurred too late after coiling. MRI reveals at this stage of inflammation but could not prove the cause of this inflammation. We doubt that probable recurrence of CCF that was not seen in MRI. So repeated angiogram was recommended, but she declined further intervention. We thought about the location of coils because coil packing in the posterior cavernous sinus can induce the abducent nerve palsy, but it didn’t explain the reason why the delayed the abducent nerve palsy occurred.
We report 2 cases of delayed cranial nerve palsy after successful coil embolization. The cause is still undefined. We should keep in mind that the delayed cranial nerve palsy could be happen after successful coil embolization of cavernous ICA aneurysm or CCF and in these cases, additional treatment such as steroid treatment can be used.
ACKNOWLEDGMENTS
This work was supported by a grant from the Chunma Medical Research Foundation, Korea, 2019.
REFERENCES
1. Bocher-Schwarz HG, Ringel K, Bohl J, Filippi R, Kempski O, Perneczky A. Histological findings in coil-packed experimental aneurysms 3 months after embolization. Neurosurgery. 2002; Feb. 50(2):379–84. discussion 384-5.


2. Bou Ghannam A, Subramanian PS. Delayed onset cranial nerve palsies after endovascular coil embolization of direct carotid-cavernous fistulas. J Neuroophthalmol. 2018; Jun. 38(2):156–9.


3. Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999; Feb. 30(2):470–6.

4. Derdeyn CP, Cross DT 3rd, Moran CJ, Brown GW, Pilgram TK, Diringer MN, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 2002; May. 96(5):837–43.


5. Kashiwazaki D, Kuwayama N, Akioka N, Kuroda S. Delayed abducens nerve palsy after transvenous coil embolization for cavernous sinus dural arteriovenous fistulae. Acta Neurochir (Wien). 2014; Jan. 156(1):97–101.


6. Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006; Nov-Dec. 27(10):2078–82.


7. Klisch J, Huppertz HJ, Spetzger U, Hetzel A, Seeger W, Schumacher M. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery. 2003; Oct. 53(4):836–56. discussion 856-7.


8. Nishino K, Ito Y, Hasegawa H, Kikuchi B, Shimbo J, Kitazawa K, et al. Cranial nerve palsy following transvenous embolization for a cavernous sinus dural arteriovenous fistula: association with the volume and location of detachable coils. J Neurosurg. 2008; Aug. 109(2):208–14.


Fig. 1.
Case 1, A 61-year-old woman with delayed incomplete Horner’s syndrome beginning 19 months following coiling of a left ICA aneurysm. (A, B) Preoperative angiography demonstrated the incidental unruptured aneurysm of the left cavernous ICA. (C, D) In postoperative angiography, complete coiling was checked. (E, F) After 19 months, follow-up angiography was done and there was no interval change on coiling site. ICA, internal carotid artery.
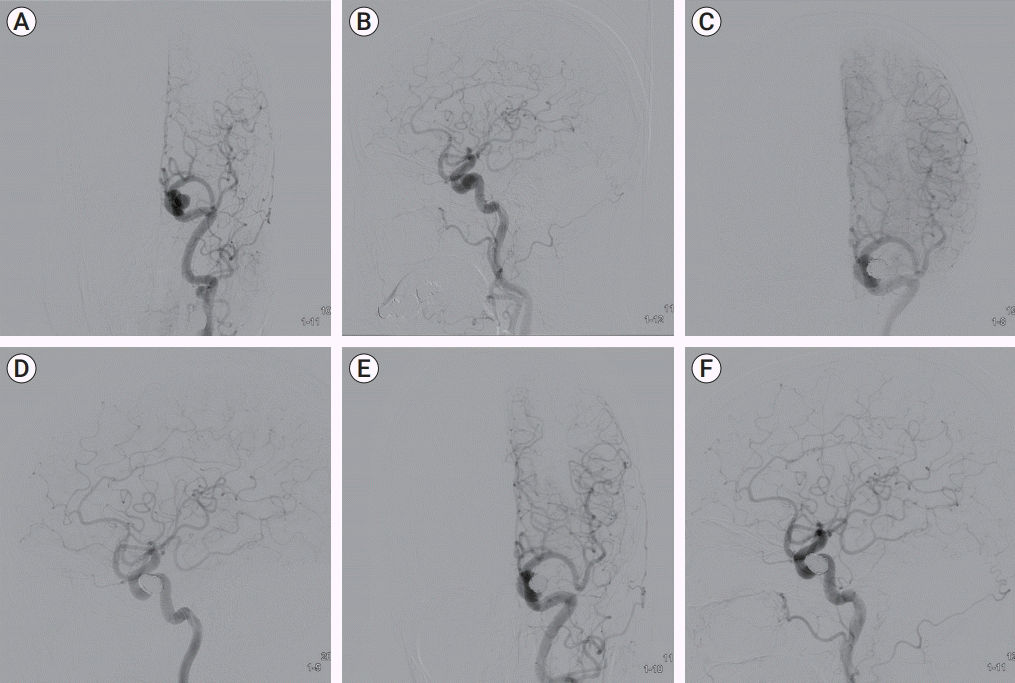
Fig. 2.
Case 1, (A) Preoperative T2-weighted axial MRI reveals an aneurysm arising from the cavernous portion of the left ICA. (B) 19 months later, postoperative T2-weighted axial MRI shows heterogeneous signal intensity from within the aneurysm, consistent with coil packing. (C, D) Postoperative T1-weighted axial MRI shows discontinuity of left CS outer wall (arrow). MRI, magnetic resonance imaging; ICA, internal carotid artery; CS, cavernous sinus.
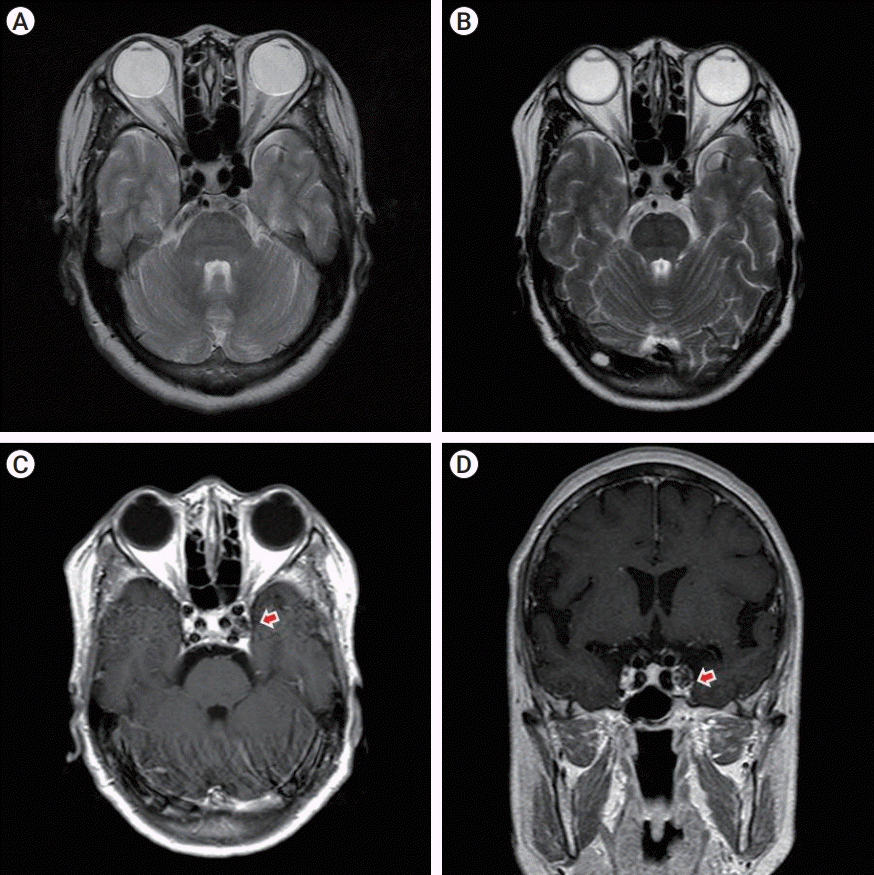
Fig. 3.
Case 2, A 50-year-old woman with delayed right 6th nerve palsy beginning 38 months after coil embolization of CCF. (A, B) Preoperative angiography was done and dural type CCF. (C, D) In postoperative angiography, CCF and shunting flow was not checked. (E, F) After 38 months, follow-up angiography was done and there was no interval change on coiling site. CCF, carotid cavernous fistula.
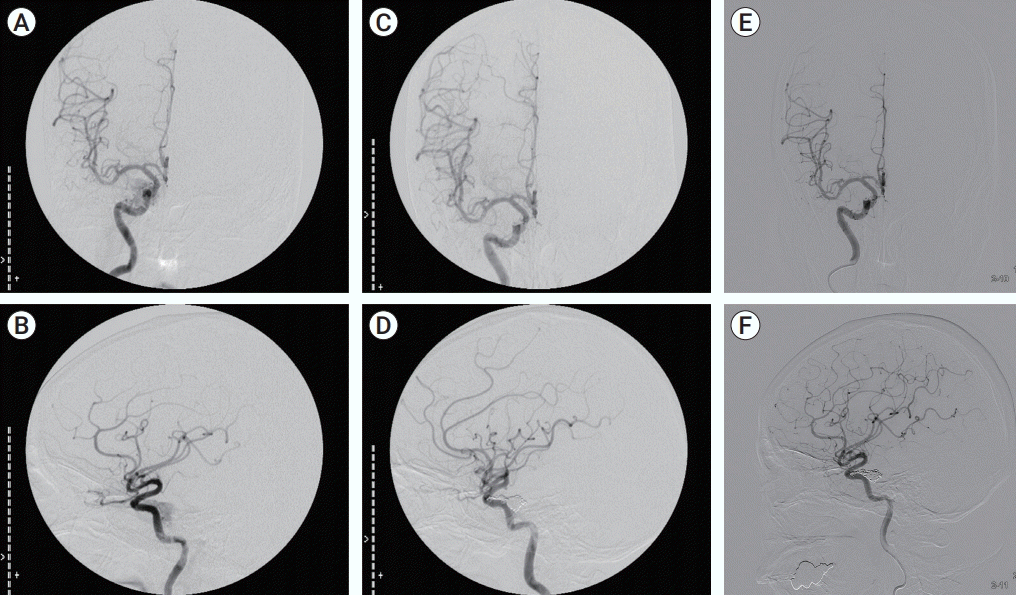
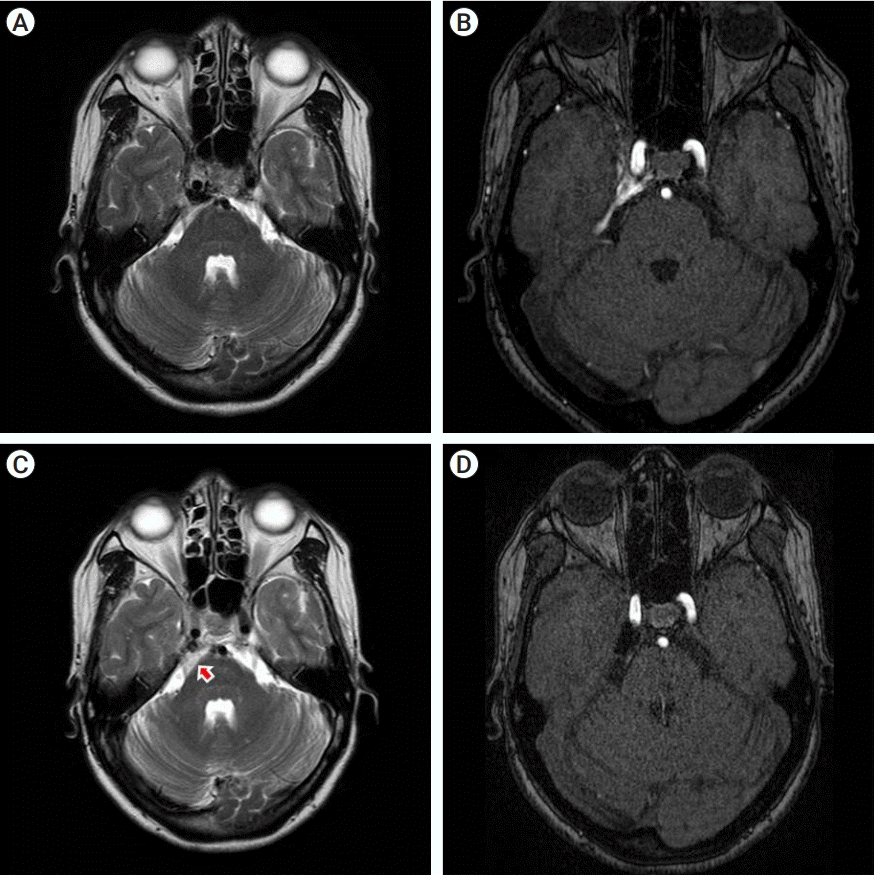
Fig. 4.
(A) Preoperative T2-weighted axial MRI shows the signal voiding on right CS. (B) preoperative MR source image shows abnormal signal flow on the right CS. (C) 38 months later, postoperative T2-weighted axial MRI shows some mixed signal intensity lesion suggesting inflammatory reaction or thrombosis (arrow) around CS and prepontine/superior cerebellopontine cistern. (D) Postoperative MR source image reveals complete obliteration of CCF. MRI, magnetic resonance imaging; CS, cavernous sinus; CCF, carotid cavernous fistula.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download