Abstract
Objective
If the size of an intracranial aneurysm is below 3 mm, clinicians rarely treat them because of the low risk of rupture. But subarachnoid hemorrhage (SAH) due to the rupture of very small intracranial aneurysm (VSIA) (saccular aneurysm sized less than 3 mm) may lead to many critical neurological complications. So we analyzed the characteristics and differences between the ruptured VSIA group and the ruptured non-VSIA group.
Methods
421 saccular aneurysms from patients with SAH between January 2016 and December 2019 were included. Patient information including age, sex, and medical history and information about the aneurysm including location, size, aspect ratio, inflow angle, and height-width ratio were collected. And we compared the VSIA group with non-VSIA group about these characteristics
Results
12.1% (51/421) of the aneurysms were included in the VSIA group, while the non-VSIA group consisted of 87.9% of the aneurysms (370/421). The female predominance was significantly higher in the VSIA group than that in the non-VSIA group (p=0.011). No significant difference was observed in location, medical history, height-width ratio between the groups. The mean value of the inflow angle in the VSIA group was much lower than that in the non-VSIA group, but no statistically significant association between rupture risk and the inflow angle was observed. The average aspect ratio was significantly lower than that in the non-VSIA group.
Conclusions
Ruptured VSIA group has higher percentage of females and lower aspect ratio than ruptured non-VSIA group. Further studies regarding the characteristics of ruptured and unruptured VSIA patients is required for assistance in clinical decision related to treatment of VSIA group before the aneurysmal sac rupture.
Intracranial aneurysms are common, with approximately 3% of prevalence. Previous studies have reported that intracranial aneurysms occur in 1-2% of the population [2]. Rupture of intracranial cerebral aneurysm result in subarachnoid hemorrhage (SAH) that may be life-threatening hemorrhage. Patients with SAH can experience several significant neurologic complications including hydrocephalus, cerebral edema, delayed ischemic stroke, rebleeding and seizure [10]. Therefore, it is essential to evaluate the rupture risk of the unruptured intracranial aneurysms. If the rupture risk of an intracranial aneurysm is high, sac obliteration using aneurysmal clipping or endovascular coil embolization is recommended. The reported annual rupture rate of intracranial aneurysms is 0.95% per year [4,8], and the rupture risk is reported to be 0.36% in intracranial aneurysms sized 3-4 mm, below 0.5% in aneurysms sized less than 5 mm [4,7]. Therefore, if the size of an intracranial aneurysm is below 3 mm, clinicians rarely treat them and opt for intermittent imaging follow-up evaluations [1]. However, SAH due to the rupture of very small intracranial aneurysm (VSIA) (saccular aneurysm sized less than 3 mm) may lead to many critical neurological complications and even death, and would be difficult to treat due to the small size of the aneurysm [5]. Therefore, we analyzed the characteristics and differences between the ruptured VSIA group and the ruptured non-VSIA group. We also tried to determine the significant characteristics that could affect the risk of rupture in the VSIA group.
In the present study, we retrospectively analyzed patients with ruptured intracranial saccular aneurysms who underwent aneurysmal clipping or endovascular coil embolization at Yeungnam University Medical Center between January 2016 and December 2019. Altogether, 421 saccular aneurysms from patients with SAH were included. Patient information including age, sex, and medical history and information about the aneurysm including location, aspect ratio, inflow angle, and height-width ratio were collected and we compared the very small intracranial aneurysm(VSIA) group with non-VSIA group about these characteristics. The calculations for the aneurysm size took into consideration the aneurysm neck width, the maximum width, and the maximum height of the aneurysm. The neck of the aneurysm was checked as the borderline connecting the aneurysmal sac with the parent artery. The height of the aneurysm was defined as the line connecting the highest point of the aneurysmal sac with the midpoint of the neck of the aneurysm. The maximum width of the aneurysm was defined as the longest length perpendicular to the height of aneurysm measured as the two sidewalls of the aneurysm (Fig. 1). The height-width ratio, the inflow angle, and the aspect ratio were determined using three-dimensional digital subtraction angiography reconstruction images. The height-width ratio was measured as the aneurysm height / the maximum width of aneurysm, the inflow angle as the angle between the parent vessel’s blood flow and the aneurysm height (Fig. 2), and the aspect ratio as the aneurysm height / the aneurysm neck width [3]. Statistical analysis was performed using IBM SPSS Statistics version 19.0. (IBM Corp., Armonk, NY, USA). Chi-squared test, and independent t-test were performed for comparison between the groups and a p value <0.05 was considered statistically significant.
Among the included sample, 12.1% (51/421) of the aneurysms were enrolled in the VSIA group, while the non-VSIA group consisted of 87.9% of the aneurysms (370/421). The mean age of the patients was 60.2 years (range: 23-92 years). The mean age of the patients in the VSIA group was 60.6 years (range: 37-87 years) and the mean age of the patients in the non-VSIA group was 60.2 years (range: 23-92 years). The proportion of females was greater in both the groups (VSIA: 42 [82.4%], non-VSIA: 239 [64.6%]). However, the female predominance was significantly higher in the VSIA group than that in the non-VSIA group (p=0.011). The location of intracranial aneurysm was divided as anterior cerebral artery (VSIA: 24 [47.0%], non-VSIA: 129 [34.9%]), middle cerebral artery (VSIA: 10 [19.6%], non-VSIA: 98 [26.5%]), internal carotid artery (paraclinoid segment, ophthalmic segment and communicating segment) (VSIA: 12 [23.6%], non-VSIA: 110 [29.7%]) and posterior circulation (posterior cerebral artery, basilar artery and vertebral artery) (VSIA: 5 [9.8%], non-VSIA: 33 [8.9%]) and there was no significant difference between two groups. No significant difference was observed in medical history between the groups (Table 1). The mean values of the height-width ratio were 1.17±0.416 and 1.15±0.461 in the VSIA group and the non-VSIA group, respectively. The difference in the height-width ratio between the groups was not statistically significant (p=0.727). The mean value of the inflow angle in the VSIA group was 48.35±31.820 degrees. It was much lower than that in the non-VSIA group (55.91±31.714 degrees). Therefore, we suspected a relationship between the rupture risk of VSIAs and the inflow angle. Unfortunately, no statistically significant association between rupture risk and the inflow angle was observed in any of the groups (p=0.148). We also divided the inflow angle into two categories: <90° and ≥90° and there was no significant difference between the groups according to these categories. There was a significant difference in the aspect ratio between the groups. The average aspect ratio was 1.32±0.498 in the VSIA group and 1.57±0.696 in the non-VSIA group. The average value of the aspect ratio in the VSIA group was significantly lower than that in the non-VSIA group (p=0.002) (Table 2).
It is known that the rupture risk of intracranial aneurysms increases with increasing size of the aneurysm [6]. Hence, patients with VSIAs are considered to be at a lower risk of rupture than non-VSIA patients. In a previous study, however, the proportion of ruptured small intracranial aneurysms sized less than 5 mm was 20.7% among all ruptured intracranial aneurysm [4]. It is known that there is other factors predicting the rupture of VSIA [6], So the risk of rupture in the VSIA group cannot be ignored even if the aneurysms are very small. Ruptured VSIAs are associated with a lower inflow angle, higher aspect ratio, and higher height-width ratio [3]. Moreover, it is known that a higher aspect ratio is associated with higher rupture risk of intracranial aneurysm [9]. Therefore, we used these parameters while deciding the treatment of unruptured intracranial aneurysms. In the present study, only the aspect ratio showed statistically significant difference between the VSIA group and the non-VSIA group. However, the mean value of the aspect ratio in the VSIA group was lower than that in the non-VSIA group. The lower value of the aspect ratio in the VSIA group may be attributed to the small size of the aneurysms. Therefore, the aspect ratio should be interpreted with caution in the VSIA group. The inflow angle of an intracranial aneurysm is a useful indicator for predicting rupture risk of intracranial aneurysm [11]. In our study, the number of patients with inflow angles less than 90 degrees was much greater than the number of patients with inflow angles more than 90 degrees in both the groups. The mean value of the inflow angle in the VSIA group was lower than that in the non-VSIA group. However, the difference was not statistically significant (p=0.111). Thus, the usefulness of the inflow angle as a significant predictive factor of the rupture risk in the VSIA group was limited. However, the inflow angle may be considered a reference for the possibility of rupture in the VSIA group. Reportedly, the rupture rate of intracranial unruptured aneurysms is higher in women [12]. In the present study, the female predominance in the VSIA group was much higher than that in the non-VSIA group. Hence, careful attention should be provided to female patients with VSIAs.
The present study has some limitations. The small study population and the single-center design of the study induced statistical limitations. Calculation of the aneurysm size in the VSIA group might be inaccurate, as the sizes of the aneurysms were too small. Thus, even a small inaccuracy in the calculated aneurysm size might have led to a considerable inaccuracy in the results. Our study had a retrospective design and it included data only from a single institution. Lastly, we didn't VSIA & non-VSIA group patients of unruptured intracranial aneurysm. If we compare the characteristics of ruptured VSIA group with unruptured VSIA group, there would be more helpful.
Ruptured VSIA group has higher percentage of females and lower aspect ratio than ruptured non-VSIA group. Further studies regarding the characteristics of ruptured and unruptured VSIA patients is required for assistance in clinical decision related to treatment of VSIA group before the aneurysmal sac rupture.
REFERENCES
2. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014; Apr. 13(4):393–404.


3. Duan Z, Li Y, Guan S, Ma C, Han Y, Ren X, et al. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep. 2018; Apr. 8(1):6440.



4. Ikawa F, Morita A, Tominari S, Nakayama T, Shiokawa Y, Date I, et al. Rupture risk of small unruptured cerebral aneurysms. J Neurosurg. 2019; Jan. 1–10.

5. Jamroz T, Jakutowicz I, Hofman M, Kolodkiewicz M, Cmiel M, Lapaj A, et al. Safety and efficacy of treatment of very small intracranial aneurysms. Pol J Radiol. 2019; Sep. 84:e360–4.
6. Lee GJ, Eom KS, Lee C, Kim DW, Kang SD. Rupture of very small intracranial aneurysms: incidence and clinical characteristics. J Cerebrovasc Endovasc Neurosurg. 2015; Sep. 17(3):217–22.



7. Malhotra A, Wu X, Forman HP, Grossetta Nardini HK, Matouk CC, Gandhi D, et al. Growth and Rupture Risk of Small Unruptured Intracranial Aneurysms: A Systematic Review. Ann Intern Med. 2017; Jul. 167(1):26–33.

8. UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012; Jun. 366(26):2474–82.


9. Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND. Is aspect ratio a reliable predictor of intracranial aneurysm rupture? Neurosurgery. 2004; Jun. 54(6):1343–7. discussion 1347-8.


10. Suarez JI. Diagnosis and Management of Subarachnoid Hemorrhage. Continuum (Minneap Minn). 2015; Oct. 21(5 Neurocritical Care):1263–87.


Fig. 1.
Three-dimensional digital subtraction angiography reconstruction image of a ruptured very small intracranial aneurysm on the anterior communicating artery. Intracranial aneurysm size was determined using the following parameters. A. Neck, B. Height, and C. Width.
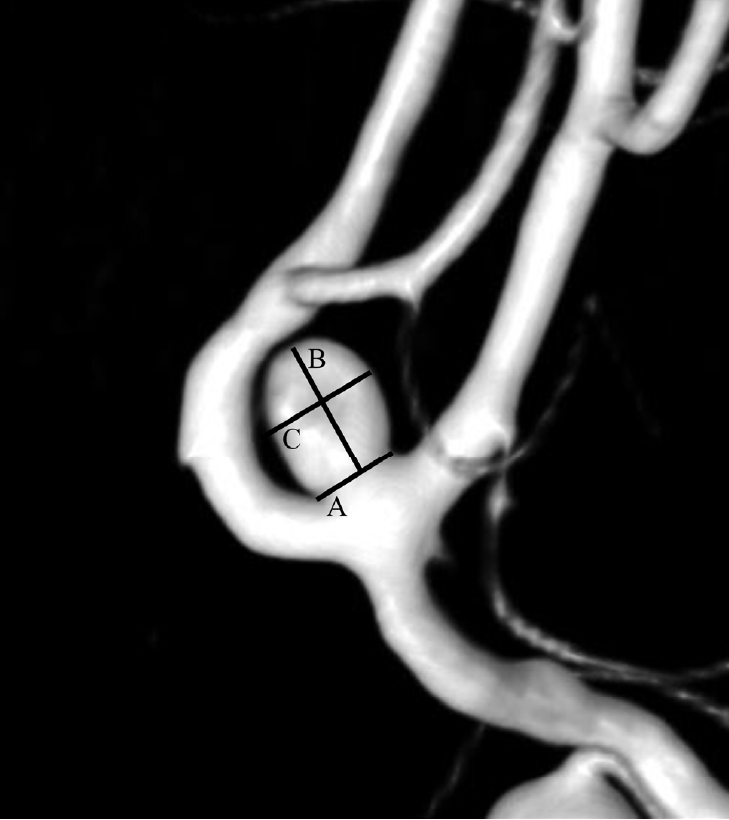
Fig. 2.
Three-dimensional digital subtraction angiography reconstruction image of a ruptured very small intracranial aneurysm. Inflow angle was defined as the angle between the parent vessel’s flow and the aneurysm height. A. inflow angle, B. parent vessel flow, C. aneurysm height direction.

Table 1.
Comparison of characteristics between VSIA group and non-VSIA group
| Variable | VSIA group (N=51) | Non-VSIA group (N=370) | p value |
|---|---|---|---|
| Sex | 0.011* | ||
| Male | 9 (17.6) | 131 (35.4) | |
| Female | 42 (82.4) | 239 (64.6) | |
| Age | 60.6 yrs (37-87) | 60.2 yrs (23-92) | 0.178 |
| Location | 0.788 | ||
| ACA | 24 (47.0) | 129 (34.9) | |
| MCA | 10 (19.6) | 98 (26.5) | |
| ICA† | 12 (23.6) | 110 (29.7) | |
| Posterior circulation‡ | 5 (9.8) | 33 (8.9) | |
| Past medical history | 0.883 | ||
| Diabetes mellitus | 3 (6) | 32 (8) | |
| Hypertension | 17 (33) | 172 (46.5) | |
| Heart disease | 1 (2) | 18 (4.9) | |
| Hyperlipidemia | 3 (5.9) | 18 (4.9) | |
| Stroke | 3 (5.9) | 16 (4.3) | |
| Smoking | 6 (11.8) | 73 (19.7) | |
| Alcohol | 11 (21.6) | 97 (26.2) | |
| Antiplatelet drug | 4 (7.8) | 28 (7.6) |
Table 2.
Comparison of height-width ratio, inflow angle, and aspect ratio between the VSIA group and the non-VSIA group
| VSIA group | Non-VSIA group | p value | |
|---|---|---|---|
| Height-width ratio (mean±SD) | 1.17±0.416 | 1.15±0.461 | 0.727 |
| Inflow angle (mean±SD) | 48.35±31.820 | 55.91±31.714 | 0.111 |
| < 90 degrees | 47 (92.2%) | 308 (83.2%) | |
| ≥ 90 degrees | 4 (7.8) | 62 (16.8) | 0.148 |
| Aspect ratio (mean±SD) | 1.32±0.498 | 1.57±0.696 | 0.002* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download