Abstract
An infrequent complication, remote cerebellar hemorrhage (RCH) may occur after supratentorial craniotomy at a reported incidence of 0.08-0.6%. Although RCH is mostly self limiting, early diagnosis and treatment are necessary as the condition may result in severe neurologic deficits or mortality. Because RCH is often asymptomatic, occurrence of it was occasionally recognized with brain computed tomography (CT) scans only. We experienced two contrasting cases of RCH in patients with unruptured cerebral aneurysms of the middle cerebral artery. These cases indicate that it should be mandatory to perform a brain CT scans immediately after surgery and on appropriate time to detect RCH. Awareness of this complication and close monitoring are essential for avoiding fatal neurological deficits or mortality.
Remote cerebellar hemorrhage (RCH) is an infrequent complication that occurs after supratentorial craniotomy; it is normally characterized by linear hemorrhages on the cerebellar surface [2-4]. Little is known about its exact underlying pathophysiologic mechanisms [7,15]. It usually shows a benign course, although it may occasionally cause serious morbidity or mortality [2,3,14,15]. Awareness and early detection of RCH would be useful to avoid any potential fatal outcomes. We experienced two cases of RCH in patients with unruptured cerebral aneurysms of the middle cerebral artery (MCA), whose clinical presentations and outcomes are different from each other. Here, we report two cases of RCH and review previous literature.
A 45-year-old man was admitted to our institution with a diagnosis of unruptured cerebral aneurysm of the right MCA, whose lesion was measured as 4.1×2.7 mm in size. The patient had no any notable medical history, and underwent surgical clipping of the aneurysm with right pterional craniotomy. He had stable hemodynamics throughout the surgery. Postoperatively, he presented with no notable symptoms and neurological deterioration other than mild headache. On routine postoperative brain computed tomography (CT) scans, however, intraventricular hemorrhage, subarachnoid hemorrhage and intracranial parenchymal hemorrhage accompanied by bilateral cerebellar edema were noted. Therefore, he was treated with intravenous mannitol and anti-hypertensive medications for strict blood pressure control, which are generally not used after the clipping of unruptured cerebral aneurysms. On postoperative day 7, the patient achieved a partial recovery from the hematoma on the cerebellum and the fourth ventricle. On postoperative day 13, he achieved a further recovery from the hematoma and was discharged 16 days after surgery without any neurological deficits (Fig. 1).
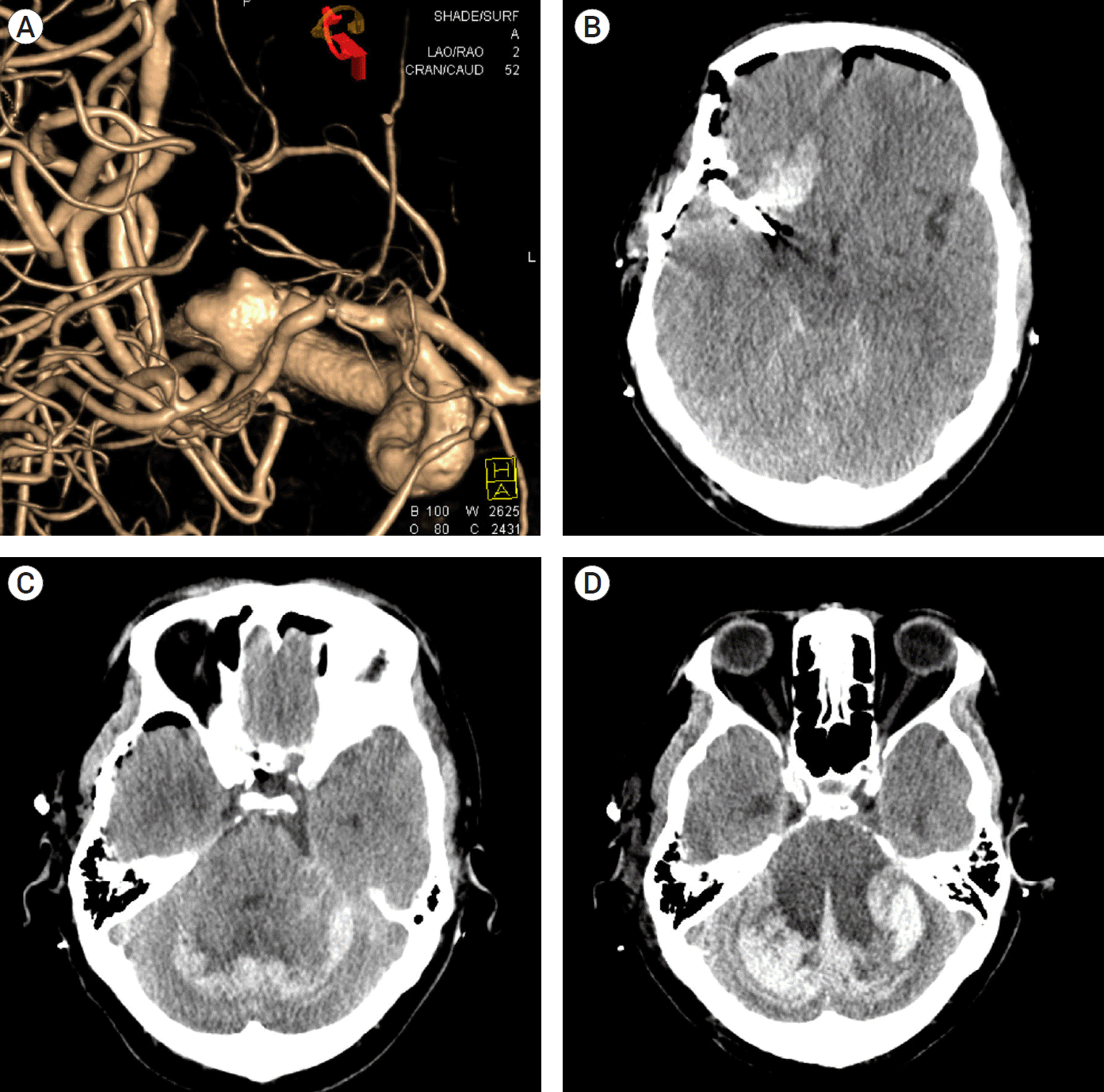
A 63-year-old man was admitted with a diagnosis of an unruptured aneurysm of the right MCA, measured as 9.5×9.2 mm in size. The patient had a medical history of hypertension and a 6-month-history of focal cerebral infarction at the right frontal lobe. After a 5-day discontinuation of an anti-platelet agent, he achieved a normalization of coagulation profile. He underwent surgical clipping of the aneurysm with right pterional craniotomy. He showed stable hemodynamics, whose systolic blood pressure was not exceed 140 mmHg during the surgery. Immediately after the surgery, the patient had RCH accompanied by bilateral cerebellar edema on brain CT scans. Then, he exhibited drowsy mental state and was treated with intravenous mannitol for intracranial pressure control and intravenous hypertensive medication for blood pressure control. Six hours thereafter, he presented with further deterioration of consciousness. Therefore, he was evaluated on brain CT scans. The follow-up brain CT scans showed an increased amount of acute intracranial hemorrhage in both cerebellar hemispheres. The patient was in need of emergent suboccipital decompression and surgery was planned, but the patient’s legal representative refused a surgical treatment. After a few hours, he presented with a sudden deterioration of the mental state to the semi-coma, accompanied by a loss of the pupillary light reflex. Within hours thereafter, the patient became hemodynamically unstable and could not even respond to inotropic agents. On postoperative day 2, the patient expired (Fig. 2).
An unusual complication, RCH occurs with an incidence of 0.08-0.6% after supratentorial surgeries and 2.8% after the clipping of unruptured intracranial aneurysm [2,4,7,14,15]. With the recently increased use of postoperative CT screening, RCH has emerged as a complication of supratentorial surgeries although it has not been well recognized [10,18].
On CT scans, RCH is characterized by subarachnoid bleeding along the cerebellar sulci, which is referred to as the “zebra sign” because of its streaky alternating density [3,8,12]. It is seen as either unilateral or bilateral to the surgical site [1]. Bilateral RCH accounts for 55% of total cases, more dominant compared with its unilateral distribution [11]. Both of our cases show typical of neuro-imaging patterns of bilateral distribution of RCH.
Little is known about the exact pathophysiology of RCH. According to a review of literature, however, there is a wide consensus on it being of venous origin and arising from an intraoperative or a postoperative loss of cerebrospinal fluid (CSF) [3,7,9,15,18]. It is generally believed that excessive CSF drainage may cause a caudal “sag” of the cerebellum with resultant stretching and tearing of the bridging veins, as they course through the cerebellar fissure where they enter the cerebellar parenchyma [6,8,14,18]. Therefore, the use of a suction drainage and intraoperative intravenous mannitol is not recommended because they may increase the risk of developing RCH [8,17]. A coagulation abnormality, arterial hypertension, venous hypertension in relation to head rotation during surgery and intraoperative opening of cistern have been proposed as risk factors for RCH [7,10,14]. However, none of these have been definitely proven to have a significant influence on development of RCH.
RCH may be clinically characterized by not only signs of increased intracranial pressure due to hydrocephalus or hematoma but also brain stem signs originated in herniation. The most common clinical presentation is mental deterioration. This may also be accompanied by such symptoms as headache, seizure, cerebellar signs and motor deficits. Otherwise, there may also be asymptomatic cases [1]. According to a recent retrospective study, RCH patients commonly present with mental alterations (31.8%), whereas a significant small number of them remain asymptomatic (23.4%) [15]. Onset of symptoms is seen within 10 hours postoperatively in 46% of total cases. In 17% of total cases, however, its onset may be delayed until ≥40 hours postoperatively. Asymptomatic cases account for almost 1/4 of the total reported cases [13,15]. In addition, onset of symptoms may be delayed. Therefore, occurrence of RCH was occasionally recognized with brain CT scans only [9]. From this context, despite a lack of notable symptoms other than mild headache in the case 1, RCH was detected on postoperative brain CT scans.
Patients with RCH usually have a good prognosis. Therefore, 32.3% of them have no residual neurological deficits. Moreover, most of the patients in this series achieved a complete recovery at a follow-up [3]. However, it has also been reported that patients with RCH are at increased risks of morbidity and mortality [8,10]. That is, approximately 9.7% of patients with RCH exhibit severe neurological deficits and 14.5% die of it [3]. A recent review of the previous published studies evaluating a total of 209 patients (134 good and 75 poor outcomes at discharge) has shown that the mortality was found to be 10.5% (21/209) prior to a follow-up [13]. According to another retrospective study, the mortality was estimated at ≥50% and 2% in patients with hydrocephalus and those without hydrocephalus, respectively [5]. Poor outcomes have been reported to be associated with complete obliteration of the cisterns. In patients with compressed cisterns, hematoma evacuation might improve outcome; yet, treatment of hydrocephalus alone does not suffice in many cases [16].
Treatment modalities are decided based on clinical findings and imaging studies. Spontaneous recovery is commonly achieved in most cases of RCH, for which conservative management is adopted [3,11]. However, there are some fatal cases of RCH, for which emergency intervention should be performed. In more detail, patients with obstructive hydrocephalus should be treated using an external ventricular drain and those with increased intracranial pressure of the posterior fossa due to hemorrhage should be treated by suboccipital decompression [2,3,5].
RCH is a rare and usually benign yet potentially fatal case of complication that occurs after supratentorial surgery. Since RCH is often asymptomatic, occurrence of RCH was occasionally recognized by brain CT scans only. Although outcomes of RCH are mostly benign, life-threatening complications that require emergent neurosurgical intervention may occur.
In conclusion, our cases indicate that it should be mandatory to perform a brain CT scans immediately after surgery, and that close monitoring and appropriate follow-up imaging studies to detect delayed RCH are essential for avoiding fatal neurological deficits or mortality.
REFERENCES
1. Amini A, Osborn AG, McCall TD, Couldwell WT. Remote cerebellar hemorrhage. AJNR Am J Neuroradiol. 2006; Feb. 27(2):387–90.

2. Baeesa SS. Remote cerebellar hemorrhage in neurosurgery. Neurosciences (Riyadh). 2012; Oct. 17(4):305–8.

3. Brockmann MA, Groden C. Remote cerebellar hemorrhage: a review. Cerebellum (London, England). 2006; Mar. 5(1):64–8.


4. Cloft HJ, Matsumoto JA, Lanzino G, Cail WS. Posterior fossa hemorrhage after supratentorial surgery. AJNR Am J Neuroradiol. 1997; Sep. 18(8):1573–80.

5. Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998; Jul. 29(7):1352–7.


6. Fischer MA, Das JM. Cerebellar hematoma. StatPearls Publishing;Treasure Island (FL): 2019.
7. Friedman JA, Piepgras DG, Duke DA, McClelland RL, Bechtle PS, Maher CO, et al. Remote cerebellar hemorrhage after supratentorial surgery. Neurosurgery. 2001; Dec. 49(6):1327–40.


8. Honegger J, Zentner J, Spreer J, Carmona H, Schulze-Bonhage A. Cerebellar hemorrhage arising postoperatively as a complication of supratentorial surgery: a retrospective study. J Neurosurg. 2002; Feb. 96(2):248–54.

9. Koller M, Ortler M, Langmayr J, Twerdy K. Posterior-fossa haemorrhage after supratentorial surgery–report of three cases and review of the literature. Acta Neurochir (Wien). 1999; 141(6):587–92.

10. König A, Laas R, Herrmann HD. Cerebellar haemorrhage as a complication after supratentorial craniotomy. Acta Neurochir (Wien). 1987; 88(3-4):104–8.

11. Liu B, Dong H, Wang J, Tian D, Li M, Liu J, et al. Remote cerebellar hemorrhage after microsurgical clipping of intracranial aneurysms: diagnosis and treatment a review of 13 cases. Int J Clin Exp Med. 2016; 9(2):3681–6.
12. Nagendran A, Patel B, Khan U. The zebra sign: an unknown known. Pract Neurol. 2016; Feb. 16(1):48–9.


13. Ott KH, Kase CS, Ojemann RG, Mohr JP. Cerebellar hemorrhage: diagnosis and treatment: a review of 56 cases. Arch Neurol. 1974; Sep. 31(3):160–7.

14. Papanastassiou V, Kerr R, Adams C. Contralateral cerebellar hemorrhagic infarction after pterional craniotomy: report of five cases and review of the literature. Neurosurgery. 1996; Oct. 39(4):841–51. discussion 851-2.


15. Park JS, Hwang JH, Park J, Hamm IS, Park YM. Remote cerebellar hemorrhage complicated after supratentorial surgery: retrospective study with review of articles. J Korean Neurosurg Soc. 2009; Aug. 46(2):136–43.



16. Van Loon J, Van Calenbergh F, Coffin J, Plets C. Controversies in the management of spontaneous cerebellar haemorrhage a consecutive series of 49 cases and review of the literature. Acta Neurochir (Wien). 1993; 122(3-4):187–93.


Fig. 1.
(A) Brain magnetic resonance angiography scans showing an unruptured cerebral aneurysm of the right middle cerebral artery. (B) Immediate postoperative non-contrast-enhanced computed tomography (CT) scans revealing subarachnoid hemorrhage in the cerebellar sulci with hemorrhage in the fourth ventricle after aneurysmal clipping. (C) Follow-up CT scans showing a recovery from the remote cerebellar hemorrhage with effacement of the fourth ventricle on postoperative day 13.
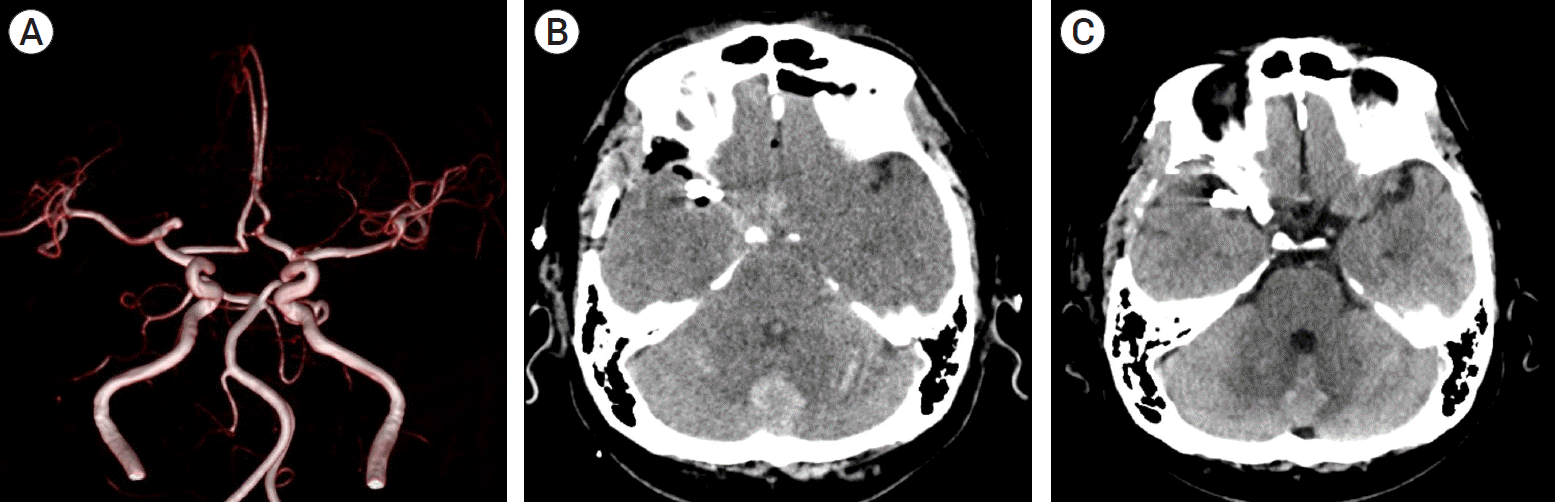
Fig. 2.
(A) Unruptured cerebral aneurysm at the bifurcation of the right middle cerebral artery on cerebral angiography. (B) The immediate postoperative non-contrast-enhanced computed tomography (CT) scans after aneurysmal clipping. (C) Immediate postoperative brain CT scans showing bilateral RCH accompanied by bilateral cerebellar edema. (D) Follow-up brain CT scans taken at six hours postoperatively revealing an increased amount of acute intracranial hemorrhage in both cerebellar hemispheres. RCH, remote cerebellar hemorrhage.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download