Abstract
The present study explored the therapeutic potential of hydrogen sulfide (H2S) in restoring aging-induced loss of cardioprotective effect of remote ischemic preconditioning (RIPC) along with the involvement of signaling pathways. The left hind limb was subjected to four short cycles of ischemia and reperfusion (IR) in young and aged male rats to induce RIPC. The hearts were subjected to IR injury on the Langendorff apparatus after 24 h of RIPC. The measurement of lactate dehydrogenase, creatine kinase and cardiac troponin served to assess the myocardial injury. The levels of H2S, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), nuclear factor erythroid 2-related factor 2 (Nrf2), and hypoxia-inducible factor (HIF-1α) were also measured. There was a decrease in cardioprotection in RIPC-subjected old rats in comparison to young rats along with a reduction in the myocardial levels of H2S, CBS, CSE, HIF-1α, and nuclear: cytoplasmic Nrf2 ratio. Supplementation with sodium hydrogen sulfide (NaHS, an H2S donor) and l-cysteine (H2S precursor) restored the cardioprotective actions of RIPC in old hearts. It increased the levels of H2S, HIF-1α, and Nrf2 ratio without affecting CBS and CSE. YC-1 (HIF-1α antagonist) abolished the effects of NaHS and l-cysteine in RIPC-subjected old rats by decreasing the Nrf2 ratio and HIF-1α levels, without altering H2S.The late phase of cardioprotection of RIPC involves an increase in the activity of H2S biosynthetic enzymes, which increases the levels of H2S to upregulate HIF-1α and Nrf2. H2S has the potential to restore aging-induced loss of cardioprotective effects of RIPC by upregulating HIF-1α/Nrf2 signaling.
Remote preconditioning is the phenomenon of inducing protection to a target organ against sustained ischemia-reperfusion injury by exposing the remote organ (non-target organ) to short non-lethal episodes of ischemia-reperfusion injury. Along with other tissues, remote preconditioning imparts cardioprotection against the harmful effects of ischemia and reperfusion [1]. In remote hind limb preconditioning, the hind limb is the remote organ, and it is subjected to short interspersed episodes of ischemia and reperfusion to induce cardioprotection. Remote hind limb preconditioning is very commonly employed in experimental studies to unfold the mechanisms involved in inducing cardioprotection during remote preconditioning [2]. Remote preconditioning induces protection in two phases, early and late phase. The early phase of protection is initiated immediately after the remote preconditioning protocol, and it persists for a relatively short period. In contrast, the late phase of protection initiates after about 24 h of remote preconditioning stimulus, and it lasts for a relatively long period. Moreover, studies have also described that the late phase of remote preconditioning confers a higher magnitude of cardioprotection than the early phase [3,4].
The applications of remote preconditioning have also been extended in clinics to protect the heart from ischemic injury, particularly during surgical procedures [5,6]. However, remote preconditioning is not always effective in imparting cardioprotection, and clinical trials have reported the negative results of remote preconditioning [7,8]. Certain diseases such as diabetes mellitus and drugs such as glibenclamide and anesthetics abolish the cardioprotective effects of remote preconditioning [9,10]. Aging is another confounding factor that attenuates the cardioprotective effects of remote preconditioning [11,12]. However, the mechanisms involved in attenuating the beneficial effects of remote preconditioning in aging hearts, particularly during the late phase, remain unexplored.
Hydrogen sulfide (H2S), a gaseous neurotransmitter, is endogenously synthesized by cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS) [13]. Recent studies have shown the diverse biological actions of H2S, including a decrease in ischemia-reperfusion injury [14]. Moreover, studies have shown that H2S also induces preconditioning-like effects to confer cardioprotection [15]. Nrf2 and HIF-1α are the transcriptional factors, and their role in inducing cardioprotection during remote preconditioning is also documented [16,17]. Furthermore, H2S triggers a signaling pathway involving the activation of Nrf2 and HIF-1α. Indeed, NaHS has been shown to exert protective effects on balloon injury-induced restenosis in the form of suppression of the neointimal hyperplasia and proliferation of human vascular smooth muscle cells via activation of Nrf2/HIF1α signaling pathway [18]. Accordingly, the present study was employed to explore the influence of aging on the late phase of cardioprotection triggered in response to remote hind limb preconditioning stimulus, the role of H2S in restoring aging-induced decrease in cardioprotective effects of remote preconditioning along with the possible role of HIF-1α and Nrf2 in H2S-triggered signaling in remote preconditioning-subjected rats.
Wistar albino male rats of 3 months age (young) and 20 months of age (old) were employed in the study [19-21]. The experimental protocol was approved by the Institutional Animal Ethics Committee of Affiliated Zhongshan Hospital of Dalian University (Ethic Number: ZN-RIB-005-02). All experiments were conducted as per ethical guidelines. The biochemical analysis was done using assay kits for LDH-1 (Elabscience, Wuhan, China), CK-MB (Elabscience, Houston, TX, USA) and CBS activity (BioVision, Inc., Milpitas, CA, USA); ELISA kits for the assay of cTnT (Cloud-Clone Corp., Katy, TX, USA), HIF-1α (Elabscience, Houston), Nrf2 (LifeSpan BioSciences, Seattle, WA, USA) and CSE (Reddot Biotech, Kelowna, BC, Canada). NaHS (Sigma Aldrich, St. Louis, MO, USA) and l-cysteine (Sigma Aldrich) were dissolved in water, while YC-1 (Sigma Aldrich) was dissolved in 10% DMSO. The doses of NaHS [22,23], l-cysteine [24] and YC-1 [25,26] were selected based on previous studies.
Animals were anesthetized with thiopental sodium (40 mg/kg, i.p.) and the left hind limb was occluded by a neonatal blood pressure cuff. The cuff was inflated up to 150 mm of Hg to induce hind limb ischemia and deflated to zero pressure to induce reperfusion to the hind limb. Four such short episodes of ischemia and reperfusion, each comprising of 5 min, constituted remote preconditioning. Since four episodes of ischemia (each of 5 min duration) and reperfusion (each of 5 min duration) were given to the hind limbs of rats to constitute remote ischemic preconditioning (RIPC) protocol, therefore, the total time of RIPC was of forty minutes. After forty minutes of remote ischemic preconditioning protocol, animals were kept for 24 h [2] and thereafter, rats were sacrificed to isolate hearts.
Twenty four hours after the last episode of remote preconditioning, the rats were sacrificed to remove the hearts. On the Langendorff apparatus, the isolated hearts were perfused with Kreb’s Henseleit (KH) solution, pH 7.4 and maintained at 37°C. KH solution was made of 118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.25 mM CaCl2, and 7 mM glucose. The hearts were initially perfused with KH solution for 15 min to allow the stabilization of heart preparation. After stabilization, inflow of KH solution to the heart was stopped to induce global ischemia for 30 min. Thereafter, the flow of KH was restored to the heart for 120 min, which constituted the reperfusion phase [27]. In short, the different phases involved stabilization (15 min), global ischemia (30 min) and reperfusion (120 min).
Ischemia-reperfusion-induced myocardial injury was quantified by measuring the release of heart-specific biochemical molecules in the coronary flow. These heart-specific biochemical molecules included LDH-1, CK-MB and cardiac troponins (cTnT).These were quantified in the coronary effluent before subjecting the heart to global ischemia and immediately after starting reperfusion with the help of commercially available kits.
The myocardial cell death in the form of infarction was quantified using triphenyl tetrazolium chloride (TTC) staining. After the completion of 120 min of reperfusion, the hearts were removed and kept in the freezer overnight. Thereafter, the frozen hearts were sliced into thin sections (2–3 mm in thickness) and these slices were placed in 1% TTC solution in 0.2 M Tris buffer (pH 7.4) at 37ºC for 20 min. Due to the leakage of the reduced form of nicotinamide adenine dinucleotide (NADH) and dehydrogenase enzyme from the dead/non-viable heart portions, the infarcted portions remained unstained in the presence of TTC. In contrast, the viable portions were stained red slices due to the presence of NADH and dehydrogenase enzymes. Thereafter, the percentage of dead/infarcted area was calculated with respect to the total area [28,29].
Nrf2 is a transcriptional factor, which is translocated to the nucleus; therefore, its levels were quantified in cytoplasm and nucleus using an ELISA kit. The nucleus and cytoplasmic fractions were separated using a nuclear/cytosol extraction kit (BioVision). The levels of Nrf2 were estimated in both nuclear and cytoplasmic fractions using a commercially available ELISA kit, based on the Sandwich assay principle, with user manual instructions. In brief, 100 μl of the sample was added to the microtiter plate, which was pre-coated with a target-specific capture antibody. Thereafter, 100 μl of detection reagent A containing a biotin-conjugated detection antibody was added and incubated for 1 h at 37°C. The unbound detection antibody was washed away. Thereafter, 100 μl of detection reagent B containing an Avidin-Horseradish peroxidase (HRP) conjugate was added and incubated for 30 min at 37°C. The unbound avidin-HRP conjugate was washed away and a tetramethyl benzidine (TMB) substrate was added that reacted with the HRP enzyme to yield a color, whose optical density was measured at a wavelength of 450 nm. The levels of HIF-1αand cystathionine-γ-lyase were also measured in the heart homogenates using commercially available ELISA kits, based on the Sandwich assay principle. The ELISA procedure for the estimation of HIF-1α and cystathionine-γ-lyase was similar to as explained for Nrf2 estimation. In these methods, 100 μl of the sample was added to specific capture antibody pre-coated 96 welled microtiter plate and thereafter, 100 μl of detection reagent A (biotin-conjugated detection antibody) and detection reagent B (avidin-HRP conjugate) were added in a sequence manner. The color was obtained by adding TMB substrate, whose absorbance was measured at a wavelength of 450 nm.
The determination of cystathionine β-synthase activity was done using a fluorometric assay kit. In this test, 40 μl of substrate solution containing cysteine and homocysteine (substrates of cystathionine β-synthase) was mixed with 30 μl of the sample (containing cystathionine β-synthase) to produce H2S. The latter was allowed to react with the azido-functional group of the fluorescent probe to yield a fluorescent amino group. The fluorescence was noted at an excitation wavelength of 368 nm and an emission wavelength of 460 nm.
Eight animals were used in each group, and a total sixteen groups were employed. The detailed protocol is explained in the form of Table 1. Briefly, the groups included normal (group I) in which rats were not subjected to any intervention and heart was isolated for biochemical estimations; control group in which isolated hearts (from young rats) were subjected to 30 min of ischemia and 120 min of reperfusion (group II); remote preconditioning in young animals (3 months old) in which remote preconditioning stimulus was given to rats and after 24 h later, hearts were isolated and subjected to 30 min of ischemia and 120 min of reperfusion (group III); remote preconditioning in aged animals in which same protocol was performed in older rats with age of 20 months (group IV); remote preconditioning in aged animals supplemented with two different doses of sodium hydrosulfide (20 and 40 μmol/kg; i.p.) in which hydrogen sulfide donor was given in aged rats along with remote preconditioning stimulus, twenty four hours before subjecting to 30 min of ischemia and 120 min of reperfusion (groups V and VI); remote preconditioning in aged animals supplemented with two different doses of l-cysteine (20 and 40 mg/kg i.p.) in which pharmacological agent was given in aged rats along with remote preconditioning stimulus, twenty four hours before subjecting to 30 min of ischemia and 120 min of reperfusion (groups VII and VIII); NaHS preconditioning in young in which sodium hydrosulfide (20 μmol/kg i.p.) was administered in young rats, twenty four hours before subjecting to 30 min of ischemia and 120 min of reperfusion (group IX); NaHS preconditioning in old in which sodium hydrosulfide (20 μmol/kg i.p.) was administered in old rats, twenty four hours before subjecting to 30 min of ischemia and 120 min of reperfusion (group X); remote preconditioning in aged animals treated with sodium hydrosulfide (20 μmol/kg i.p.) and YC-1 (1 mg/kg i.p.) (group XI); remote preconditioning in aged animals treated with sodium hydrosulfide (20 μmol/kg i.p.) and YC-1 (2 mg/kg i.p.) (group XII); remote preconditioning in aged animals treated with l-cysteine (20 mg/kg i.p.) and YC-1 (1 mg/kg i.p.) (group XIII); remote preconditioning in aged animals treated with l-cysteine (20 mg/kg i.p.) and YC-1 (2 mg/kg i.p.) (group XIV); remote preconditioning in aged animals treated with sodium hydrosulfide (20 μmol/kg i.p.) and DMSO (solvent of YC-1) (group XV); remote preconditioning in aged animals treated with l-cysteine (20 mg/kg i.p.) and DMSO (solvent of YC-1) (group XVI).
Statistical analyses were performed using GraphPad Prism 7 (GraphPad software Inc., San Diego, CA, USA). The data were presented in the form of mean ± standard deviation. Two way repeated measure ANOVA was employed to compare the data of LDH-1, CK-MB, and cTnT. One-way ANOVA was employed to compare the data of infarct size, H2S, CBS, CSE, Nrf-2, and HIF-1α. Tukey’s test was employed for post-hoc analysis. Statistical significance was calculated by fixing p < 0.05.
There was a marked increase in the release of LDH-1 (Fig. 1A), CK-MB (Fig. 1B), and cTnT (Fig. 1C) from the myocardium into the coronary effluent after 30 min of global ischemia i.e., during the reperfusion phase in comparison to pre-ischemic state i.e., before subjecting to ischemia. There was also a significant development of myocardial infarction in ischemia-reperfusion subjected isolated hearts (Fig. 2). Short episodes of ischemia and reperfusion to the hind limb in the form of remote preconditioning triggered late cardioprotection in ischemia-reperfusion subjected young rats as assessed by a significant decrease in the release of LDH-1, CK-MB, and cTnT along with a reduction in the infarct size. Interestingly, the late cardioprotective effects of remote preconditioning observed after 24 h of its application were significantly abolished in old or aging rats. In other words, remote preconditioning conferred cardioprotection against ischemia-reperfusion injury only in young, not in old aging rat hearts.
There was a significant decrease in the levels of CBS, CSE, and H2S in rat hearts subjected to ischemia-reperfusion injury. Remote preconditioning significantly restored the levels of H2S and its biosynthetic enzymes in young rat hearts. However, the restorative effects of remote preconditioning were not observed in aging rat hearts suggesting that the failure to restore the levels of H2S may be the contributory mechanism in abolishing the cardioprotective effects of remote preconditioning in old rats. Nevertheless, the attenuated effects of remote preconditioning in aging rat hearts were significantly restored on supplementation with NaHS (20 and 40 μmol/kg), an H2S donor, and l-cysteine (20 and 40 mg/kg), an H2S precursor. These pharmacological agents restored the cardioprotective effects of remote preconditioning in aged rats in a significant manner without any significant effect on the levels of CBS (Fig. 3A) and CSE (Fig. 3B) in rat hearts. However, there was a significant restoration of the H2S levels (Fig. 4). Moreover, per se administration of NaHS (without RIPC) in the form of preconditioning also produced cardioprotection in young and old rats. However, the effects of NaHS preconditioning were significantly less as compared to a combination of RIPC and NaHS. It is important to note that in the present study, no dose-dependent effects of NaHS (20 and 40 μmol/kg) and l-cysteine (20 and 40 mg/kg) were observed. The lack of additional effects of these pharmacological agents with higher doses indicates that the optimal effects were already produced with lower doses. In other words, the selected doses may be on the higher side of the dose-response curve. Therefore, the lower doses of NaHS (20 μmol/kg) and l-cysteine (20 mg/kg) were used in subsequent groups (IX, X, XI, XII, XIII, XIV, XV, XVI) to explore the interrelationship between H2S and HIF-1α in remote preconditioning-subjected old rats.
Nrf2 is a transcriptional factor, which is translocated from the cytoplasm to the nucleus. Remote preconditioning led to an increase in the nuclear: cytosolic ratio of Nrf2 in ischemia-reperfusion-subjected young rat hearts, which was significantly abolished in old rat hearts (Fig. 5A). Moreover, there was a significant restoration of ischemia-reperfusion-induced decrease in HIF-1α levels (Fig. 5B) in response to remote preconditioning stimulus in young rat hearts. However, there was no such restoration was observed in old rat hearts subjected to remote preconditioning and the levels of HIF-1α remained low. Supplementation with NaHS (20 and 40 μmol/kg) and l-cysteine (20 and 40 mg/kg) restored the effects of remote preconditioning and there was a significant upregulation of nuclear: cytosolic ratio of Nrf-2 and HIF-1α levels in the aging hearts. Administration of YC-1, a selective inhibitor of HIF-1α, (1 and 2 mg/kg) abolished the restorative effects of NaHS and l-cysteine in remote preconditioning subjected old rats and there was a significant increase in the LDH-1, CK-MB, cTnT and infarct size. Moreover, NaHS and l-cysteine-mediated restorative effects of remote preconditioning on Nrf-2 ratio and HIF-1α levels were abolished in the presence of YC-1. However, it did not affect the levels of H2S, CBS and CSE in remote preconditioning-subjected old rats supplemented with NaHS and l-cysteine. Administration of DMSO (solvent of YC-1) did not influence the effects of sodium hydrosulfide and l-cysteine in remote preconditioning-subjected old rats.
In the present investigation, there was a significant increase in the myocardial injury in the control group in response to 30 min of global ischemia and 120 min of reperfusion. The myocardial injury was assessed by measuring the levels of LDH-1, CK-MB and cTnT in the coronary effluent. LDH-1 and CK-MB are the heart-specific enzymes and their release from the myocardium is indicative of myocardial injury. cTnT is another specific biomarker of heart injury and its release is directly correlated with heart injury [33,34]. Moreover, there was a significant increase in myocardial infarction as assessed by TTC stain in control in comparison to the normal group (Figs. 2 and 6). Brief episodes of ischemia and reperfusion (5 min each) to the hind limb conferred the cardioprotection in the form of remote preconditioning. The cardioprotective potential of remote preconditioning was assessed after 24 h of remote preconditioning stimulus i.e., during the second window of protection. Indeed, remote preconditioning is shown to confer cardioprotection in two phases i.e. an early phase (first window) and late phase (second window) [3,35]. These observations of the present study documenting the myocardial injury in response to ischemia and reperfusion along with the cardioprotective effects of the late phase of remote preconditioning are in accordance with the previously published studies [4,36].
Moreover, the present study results document that the cardioprotective effects of the late phase of remote preconditioning were significantly attenuated in old rats. It suggests that aging has a negative influence on the cardioprotective effects of remote preconditioning. There have been studies documenting that the cardioprotective potential of remote preconditioning is influenced by various factors, including concurrent medications, anesthetics or co-morbidities such as angina pectoris and diabetes mellitus [37,38]. Similarly, aging is another confounding factor that negatively influences the beneficial outcomes of remote preconditioning [11,39,40]. Indeed, it has been shown that the cardioprotective effects of RIPC are abolished in aged rats [39]. However, to the best of our knowledge, it is the first report documenting that the cardioprotection offered during the late phase of remote preconditioning is abolished in aged rat hearts. From the studies obtained from young and old aged humans volunteers subjected to RIPC, it was shown that the RIPC plasma of aged volunteers failed to confer cardioprotective effects, which was in contrast to RIPC plasma of young volunteers. Accordingly, it was proposed that there may be a decline in the release of cardioprotective humoral factors in response to RIPC due to aging [40].
To explore the mechanisms involved in attenuating the cardioprotective effects of remote preconditioning in aged hearts, the levels of H2S along with its biosynthetic enzymes, CBS and CSE, were measured in the rat hearts. There was a significant decrease in the levels of H2S, CBS and CSE in rat hearts subjected to ischemia-reperfusion injury, which was restored in remote preconditioning-subjected young rats. However, remote preconditioning failed to restore ischemia-reperfusion-induced decrease in the levels of H2S, CBS, and CSE in old aged rat hearts. These observations suggest that the cardioprotective effects of remote preconditioning during the late phase may be dependent on the increase in the levels of H2S and its biosynthetic enzymes. The protective effects of H2S preconditioning on the heart and other organs in ischemia-reperfusion-subjected animals have been documented [15,38,39,41,42]. However, it is the first study showing that the late phase of cardioprotective effects of remote preconditioning is mediated through an increase in the levels of H2S. Another hypothesis may be postulated on the basis of these observations that the cardioprotective effects of remote preconditioning in aged rats' hearts may be weaned off due to their decreased ability to raise the levels of H2S. This contention is supported by the findings of the present study showing the restoration of cardioprotective effects of remote preconditioning along with the levels of H2S in aging hearts in the presence of H2S donor (NaHS) or H2S precursor (l-cysteine).
In the present study, the significant role of Nrf2 and HIF-1α was also noted in attenuating the cardioprotective effects of remote preconditioning in aging rats. Remote preconditioning failed to restore ischemia-reperfusion-induced decrease in the HIF-1α levels in old rat hearts in comparison to young rats. Moreover, remote preconditioning-induced increase in the nuclear: cytosolic ratio of Nrf2 in ischemia-reperfusion-subjected young rat hearts was significantly abolished in old rat hearts. Nrf2 is a transcriptional factor, which exerts antioxidant actions and its levels are significantly decreased with aging [43]. Furthermore, studies have also documented the age-dependent decrease in HIF-1α levels and associated functions [44,45]. However, exogenous administration of H2S donor (NaHS) and precursor (l-cysteine) increased the nuclear: cytosolic ratio of Nrf2 in ischemia-reperfusion subjected hearts. Moreover, these interventions restored ischemia-reperfusion-induced decrease in HIF-1α levels in remote preconditioning-subjected old rat hearts. It suggests that changes in the Nrf2 ratio and HIF-1α levels in remote preconditioning rats may be secondary to the decrease in H2S levels. Accordingly, an increase in the levels of H2S (by NaHS or l-cysteine) may restore the levels of transcriptional factors. There have been earlier studies showing that the H2S-triggered signaling cascade in different tissues, including the heart involves the upregulation of transcriptional factors including Nrf2 and HIF-1α [46,47]. The significant role of HIF-1α in H2S-mediated restoration of cardioprotective effects of remote preconditioning in old rat hearts was further demonstrated in the present study showing that a selective HIF-1α inhibitor abolished the effects of NaHS and l-cysteine in remote preconditioning-subjected old rats. In other words, NaHS and l-cysteine failed to restore the cardioprotective effects of remote preconditioning in old rat hearts in the presence of HIF-1α inhibitor. It is also worth mentioning that HIF-1α inhibitor abolished the effects of NaHS and l-cysteine in remote preconditioning-subjected old rats by decreasing the nuclear: cytoplasmic Nrf2 ration and HIF-1α levels, without any significant effect on the levels of H2S, CBS and CSE. The present study results describing the changes in the Nrf2 ratio in the presence of HIF-1α inhibitor suggests the close interrelationship between HIF-1α and Nrf2, which is supported by the results of previous studies [18,48,49]. Both HIF-1 and Nrf2 are activated in response to hypoxia and these induce changes in the cell to combat the deleterious effects of hypoxia. Even in the tumor cells, there is coordination between HIF-1 and Nrf2, which is critical for tumor survival and progression [48]. A cross talk has also been identified between Nrf2 and HIF upon exposure of HEK293 cells to diverse forms of redox therapeutics [49].
Accordingly, it may be proposed that the late phase of cardioprotective effects of remote preconditioning may involve an increase in the activity of H2S biosynthetic enzymes, which may increase the levels of H2S in the myocardium to up-regulate the expression of HIF-1α and nuclear: cytoplasmic ration of Nrf2 in young hearts. However, a decrease in the ability to increase the CBS, CSE, H2S along with HIF-1α and Nrf2 ratio may decrease the cardioprotective imparting actions of remote preconditioning in aging rats.
There is a significant loss of cardioprotective potential of remote preconditioning during the delayed phase in aging rats. The failure of remote preconditioning to induce cardioprotection may be possibly due to reduced enzymatic activities of H2S biosynthetic enzymes, which eventually results in decreased H2S-triggered signaling pathway involving HIF-1α and Nrf2. Supplementation of H2S may restore aging-induced loss of cardioprotective effects of remote preconditioning by upregulating the HIF-1α and Nrf2 signaling pathway.
Notes
REFERENCES
1. Donato M, Evelson P, Gelpi RJ. 2017; Protecting the heart from ischemia/reperfusion injury: an update on remote ischemic preconditioning and postconditioning. Curr Opin Cardiol. 32:784–790. DOI: 10.1097/HCO.0000000000000447. PMID: 28902715.

2. Singh A, Randhawa PK, Bali A, Singh N, Jaggi AS. 2017; Exploring the role of TRPV and CGRP in adenosine preconditioning and remote hind limb preconditioning-induced cardioprotection in rats. Cardiovasc Drugs Ther. 31:133–143. DOI: 10.1007/s10557-017-6716-3. PMID: 28194544.


3. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. 2005; Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 46:450–456. DOI: 10.1016/j.jacc.2005.04.044. PMID: 16053957.

4. Singh H, Kumar M, Singh N, Jaggi AS. 2019; Late phases of cardioprotection during remote ischemic preconditioning and adenosine preconditioning involve activation of neurogenic pathway. J Cardiovasc Pharmacol. 73:63–69. DOI: 10.1097/FJC.0000000000000634. PMID: 30422893.


5. Ravingerova T, Farkasova V, Griecsova L, Carnicka S, Murarikova M, Barlaka E, Kolar F, Bartekova M, Lonek L, Slezak J, Lazou A. 2016; Remote preconditioning as a novel "conditioning" approach to repair the broken heart: potential mechanisms and clinical applications. Physiol Res. 65 Suppl 1:S55–S64. DOI: 10.33549/physiolres.933392. PMID: 27643940.

6. Lau JK, Roy P, Javadzadegan A, Moshfegh A, Fearon WF, Ng M, Lowe H, Brieger D, Kritharides L, Yong AS. 2018; Remote ischemic preconditioning acutely improves coronary microcirculatory function. J Am Heart Assoc. 7:e009058. DOI: 10.1161/JAHA.118.009058. PMID: 30371329. PMCID: PMC6404904.

7. Moscarelli M, Fiorentino F, Suleiman MS, Emanueli C, Reeves BC, Punjabi PP, Angelini GD. 2019; Remote ischaemic preconditioning in isolated aortic valve and coronary artery bypass surgery: a randomized trial†. Eur J Cardiothorac Surg. 55:905–912. DOI: 10.1093/ejcts/ezy404. PMID: 30544237. PMCID: PMC6477640.


8. Antonowicz SS, Cavallaro D, Jacques N, Brown A, Wiggins T, Haddow JB, Kapila A, Coull D, Walden A. 2018; Remote ischemic preconditioning for cardioprotection in elective inpatient abdominal surgery - a randomized controlled trial. BMC Anesthesiol. 18:76. DOI: 10.1186/s12871-018-0524-6. PMID: 29945555. PMCID: PMC6020340.



9. Cho YJ, Nam K, Kim TK, Choi SW, Kim SJ, Hausenloy DJ, Jeon Y. 2019; Sevoflurane, propofol and carvedilol block myocardial protection by limb remote ischemic preconditioning. Int J Mol Sci. 20:269. DOI: 10.3390/ijms20020269. PMID: 30641885. PMCID: PMC6359553.


10. Bunte S, Behmenburg F, Eckelskemper F, Mohr F, Stroethoff M, Raupach A, Heinen A, Hollmann MW, Huhn R. 2019; Cardioprotection by humoral factors released after remote ischemic preconditioning depends on anesthetic regimen. Crit Care Med. 47:e250–e255. DOI: 10.1097/CCM.0000000000003629. PMID: 30608281.

11. Lou B, Gao H, Zhou C. 2017; Myocardial protection by remote ischemic preconditioning in elective PCI: effect of ageing. Int J Cardiol. 243:105. DOI: 10.1016/j.ijcard.2017.03.142. PMID: 28747017.


12. Randhawa PK, Bali A, Virdi JK, Jaggi AS. 2018; Conditioning-induced cardioprotection: aging as a confounding factor. Korean J Physiol Pharmacol. 22:467–479. DOI: 10.4196/kjpp.2018.22.5.467. PMID: 30181694. PMCID: PMC6115349.



13. Kabil O, Banerjee R. 2014; Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 20:770–782. DOI: 10.1089/ars.2013.5339. PMID: 23600844. PMCID: PMC3910450.



14. Wu D, Wang J, Li H, Xue M, Ji A, Li Y. 2015; Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid Med Cell Longev. 2015:186908. DOI: 10.1155/2015/186908. PMID: 26064416. PMCID: PMC4443900.

15. Li C, Hu M, Wang Y, Lu H, Deng J, Yan X. 2015; Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int J Clin Exp Pathol. 8:7740–7751. PMID: 26339339. PMCID: PMC4555667.


16. Zhou C, Li H, Yao Y, Li L. 2014; Delayed remote ischemic preconditioning produces an additive cardioprotection to sevoflurane postconditioning through an enhanced heme oxygenase 1 level partly via nuclear factor erythroid 2-related factor 2 nuclear translocation. J Cardiovasc Pharmacol Ther. 19:558–566. DOI: 10.1177/1074248414524479. PMID: 24651515.


17. Kalakech H, Tamareille S, Pons S, Godin-Ribuot D, Carmeliet P, Furber A, Martin V, Berdeaux A, Ghaleh B, Prunier F. 2013; Role of hypoxia inducible factor-1α in remote limb ischemic preconditioning. J Mol Cell Cardiol. 65:98–104. DOI: 10.1016/j.yjmcc.2013.10.001. PMID: 24140799.


18. Ling K, Xu A, Chen Y, Chen X, Li Y, Wang W. 2019; Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1α signaling pathway. Int J Mol Med. 43:1299–1310. DOI: 10.3892/ijmm.2019.4076. PMID: 30747216. PMCID: PMC6365080.



19. Mesbahzadeh B, Salarjavan H, Samarghandian S, Farkhondeh T. 2021; Chlorpyrifos with age-dependent effects in cardiac tissue of male rats. Curr Mol Pharmacol. doi: 10.2174/1874467214666210111105321. [Epub ahead of print]. DOI: 10.2174/1874467214666210111105321. PMID: 33430739.

20. Wen J, Chen Z, Wang S, Zhao M, Wang S, Zhao S, Zhang X. 2021; Age-related reductions in the excitability of phasic dorsal root ganglion neurons innervating the urinary bladder in female rats. Brain Res. 1752:147251. DOI: 10.1016/j.brainres.2020.147251. PMID: 33421375.

21. Kim KW, Cho HJ, Khaliq SA, Son KH, Yoon MS. 2020; Comparative analyses of mTOR/Akt and muscle atrophy-related signaling in aged respiratory and gastrocnemius muscles. Int J Mol Sci. 21:2862. DOI: 10.3390/ijms21082862. PMID: 32326050. PMCID: PMC7215274.


22. Gao C, Xu DQ, Gao CJ, Ding Q, Yao LN, Li ZC, Chai W. 2012; An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor κB inhibitor kinase/nuclear factor κb inhibitor/nuclear factor-κB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull. 35:1029–1034. DOI: 10.1248/bpb.b110679. PMID: 22791148.

23. Zhang XJ, Meng XY, Huang XL, Dai HY, Wei P, Ling YL. 2011; [Administration of hydrogen sulfide intraperitoneally reverses the hyporesponsiveness of rat pulmonary artery induced by lipopolysaccharide and its relationship with carbon monoxide]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:200–203. Chinese. PMID: 21473819.

24. Yang LJ, Wan R, Shen JQ, Shen J, Wang XP. 2013; Effect of L-cysteine on remote organ injury in rats with severe acute pancreatitis induced by bile-pancreatic duct obstruction. Hepatobiliary Pancreat Dis Int. 12:428–435. DOI: 10.1016/S1499-3872(13)60067-3. PMID: 23924502.


25. Yan J, Zhou B, Taheri S, Shi H. 2011; Differential effects of HIF-1 inhibition by YC-1 on the overall outcome and blood-brain barrier damage in a rat model of ischemic stroke. PLoS One. 6:e27798. DOI: 10.1371/journal.pone.0027798. PMID: 22110762. PMCID: PMC3218033.

26. Komsuoglu Celikyurt I, Utkan T, Ozer C, Gacar N, Aricioglu F. 2014; Effects of YC-1 on learning and memory functions of aged rats. Med Sci Monit Basic Res. 20:130–137. DOI: 10.12659/MSMBR.891064. PMID: 25144469. PMCID: PMC4148360.



27. Dong L, Fan Y, Shao X, Chen Z. 2011; Vitexin protects against myocardial ischemia/reperfusion injury in Langendorff-perfused rat hearts by attenuating inflammatory response and apoptosis. Food Chem Toxicol. 49:3211–3216. DOI: 10.1016/j.fct.2011.09.040. PMID: 22001368.


28. Diwan V, Kant R, Jaggi AS, Singh N, Singh D. 2008; Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 315:195–201. DOI: 10.1007/s11010-008-9808-3. PMID: 18528635.

29. Kakimoto Y, Tsuruyama T, Miyao M, Abiru H, Sumiyoshi S, Kotani H, Haga H, Tamaki K. 2013; The effectiveness and limitations of triphenyltetrazolium chloride to detect acute myocardial infarction at forensic autopsy. Am J Forensic Med Pathol. 34:242–247. DOI: 10.1097/PAF.0b013e31828879cd. PMID: 23949140.


30. Xu Z, Prathapasinghe G, Wu N, Hwang SY, Siow YL, O K. 2009; Ischemia-reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. Am J Physiol Renal Physiol. 297:F27–F35. DOI: 10.1152/ajprenal.00096.2009. PMID: 19439522.
31. Siegel LM. 1965; A direct microdetermination for sulfide. Anal Biochem. 11:126–132. DOI: 10.1016/0003-2697(65)90051-5. PMID: 14328633.

32. Stipanuk MH, Beck PW. 1982; Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 206:267–277. DOI: 10.1042/bj2060267. PMID: 7150244. PMCID: PMC1158582.



33. Sadoh WE, Eregie CO, Nwaneri DU, Sadoh AE. 2014; The diagnostic value of both troponin T and creatinine kinase isoenzyme (CK-MB) in detecting combined renal and myocardial injuries in asphyxiated infants. PLoS One. 9:e91338. DOI: 10.1371/journal.pone.0091338. PMID: 24625749. PMCID: PMC3953387.

34. Amani M, Jeddi S, Ahmadiasl N, Usefzade N, Zaman J. 2013; Effect of HEMADO on level of CK-MB and LDH enzymes after ischemia/reperfusion injury in isolated rat heart. Bioimpacts. 3:101–104. DOI: 10.5681/bi.2013.003. PMID: 23878794. PMCID: PMC3713869.

35. Tapuria N, Junnarkar S, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. 2012; Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning. HPB (Oxford). 14:87–97. DOI: 10.1111/j.1477-2574.2011.00407.x. PMID: 22221569. PMCID: PMC3277050.



36. Song Y, Ye YJ, Li PW, Zhao YL, Miao Q, Hou DY, Ren XP. 2016; The cardioprotective effects of late-phase remote preconditioning of trauma depends on neurogenic pathways and the activation of PKC and NF-κB (but not iNOS) in mice. J Cardiovasc Pharmacol Ther. 21:310–319. DOI: 10.1177/1074248415609435. PMID: 26450997.


37. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. 2014; Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 66:1142–1174. DOI: 10.1124/pr.113.008300. PMID: 25261534.


38. Przyklenk K. 2011; Efficacy of cardioprotective 'conditioning' strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 28:331–343. DOI: 10.2165/11587190-000000000-00000. PMID: 21542657.

39. Behmenburg F, Heinen A, Bruch LV, Hollmann MW, Huhn R. 2017; Cardioprotection by remote ischemic preconditioning is blocked in the aged rat heart in vivo. J Cardiothorac Vasc Anesth. 31:1223–1226. DOI: 10.1053/j.jvca.2016.07.005. PMID: 27793521.


40. Heinen A, Behmenburg F, Aytulun A, Dierkes M, Zerbin L, Kaisers W, Schaefer M, Meyer-Treschan T, Feit S, Bauer I, Hollmann MW, Huhn R. 2018; The release of cardioprotective humoral factors after remote ischemic preconditioning in humans is age- and sex-dependent. J Transl Med. 16:112. DOI: 10.1186/s12967-018-1480-0. PMID: 29703217. PMCID: PMC5921545.



41. Ji K, Xue L, Cheng J, Bai Y. 2016; Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res Bull. 121:68–74. DOI: 10.1016/j.brainresbull.2015.12.007. PMID: 26772627.

42. Pan TT, Chen YQ, Bian JS. 2009; All in the timing: a comparison between the cardioprotection induced by H2S preconditioning and post-infarction treatment. Eur J Pharmacol. 616:160–165. DOI: 10.1016/j.ejphar.2009.05.023. PMID: 19482017.

43. Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T. 2016; Repression of the antioxidant NRF2 pathway in premature aging. Cell. 165:1361–1374. DOI: 10.1016/j.cell.2016.05.017. PMID: 27259148. PMCID: PMC4893198.



44. Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. 2000; Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 275:29643–29647. DOI: 10.1074/jbc.M001029200. PMID: 10882714.

45. Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. 2007; Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 116:2818–2829. DOI: 10.1161/CIRCULATIONAHA.107.715847. PMID: 18040029.

46. Shimizu Y, Nicholson CK, Lambert JP, Barr LA, Kuek N, Herszenhaut D, Tan L, Murohara T, Hansen JM, Husain A, Naqvi N, Calvert JW. 2016; Sodium sulfide attenuates ischemic-induced heart failure by enhancing proteasomal function in an Nrf2-dependent manner. Circ Heart Fail. 9:e002368. DOI: 10.1161/CIRCHEARTFAILURE.115.002368. PMCID: PMC4826721. PMID: 27056879.

47. Lohninger L, Tomasova L, Praschberger M, Hintersteininger M, Erker T, Gmeiner BM, Laggner H. 2015; Hydrogen sulphide induces HIF-1α and Nrf2 in THP-1 macrophages. Biochimie. 112:187–195. DOI: 10.1016/j.biochi.2015.03.009. PMID: 25795259.

48. Toth RK, Warfel NA. 2017; Strange bedfellows: nuclear factor, erythroid 2-like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in tumor hypoxia. Antioxidants (Basel). 6:27. DOI: 10.3390/antiox6020027. PMID: 28383481. PMCID: PMC5488007.


49. Johansson K, Cebula M, Rengby O, Dreij K, Carlström KE, Sigmundsson K, Piehl F, Arnér ES. 2017; Cross talk in HEK293 cells between Nrf2, HIF, and NF-κB activities upon challenges with redox therapeutics characterized with single-cell resolution. Antioxid Redox Signal. 26:229–246. DOI: 10.1089/ars.2015.6419. PMID: 26415122. PMCID: PMC5704776.



Fig. 1
Influence of remote ischemic preconditioning (RIPC), hydrogen sulfide (H2S) donor, H2S precursor and HIF-1α inhibitor on ischemia-reperfusion-induced LDH-1 (A), CK-MB (B) and cTnT (C) release in young and old rats.
The levels were measured in the coronary effluent immediately before subjecting to ischemia and immediately after initiating reperfusion. HIF, hypoxia-inducible factor; LDH, lactate dehydrogenase; CK, creatine kinase; cTnT, cardiac troponin T; NaHS, sodium hydrosulfide; LC, l-cysteine. ap < 0.05 vs. control before ischemia; bp < 0.05 vs. control during reperfusion; cp < 0.05 vs. RIPC in young; dp < 0.05 vs. RIPC in old; ep < 0.05 vs. NaHS (20 µM/kg) and RIPC in old; fp < 0.05 vs. LC (20 mg/kg) and RIPC in old.
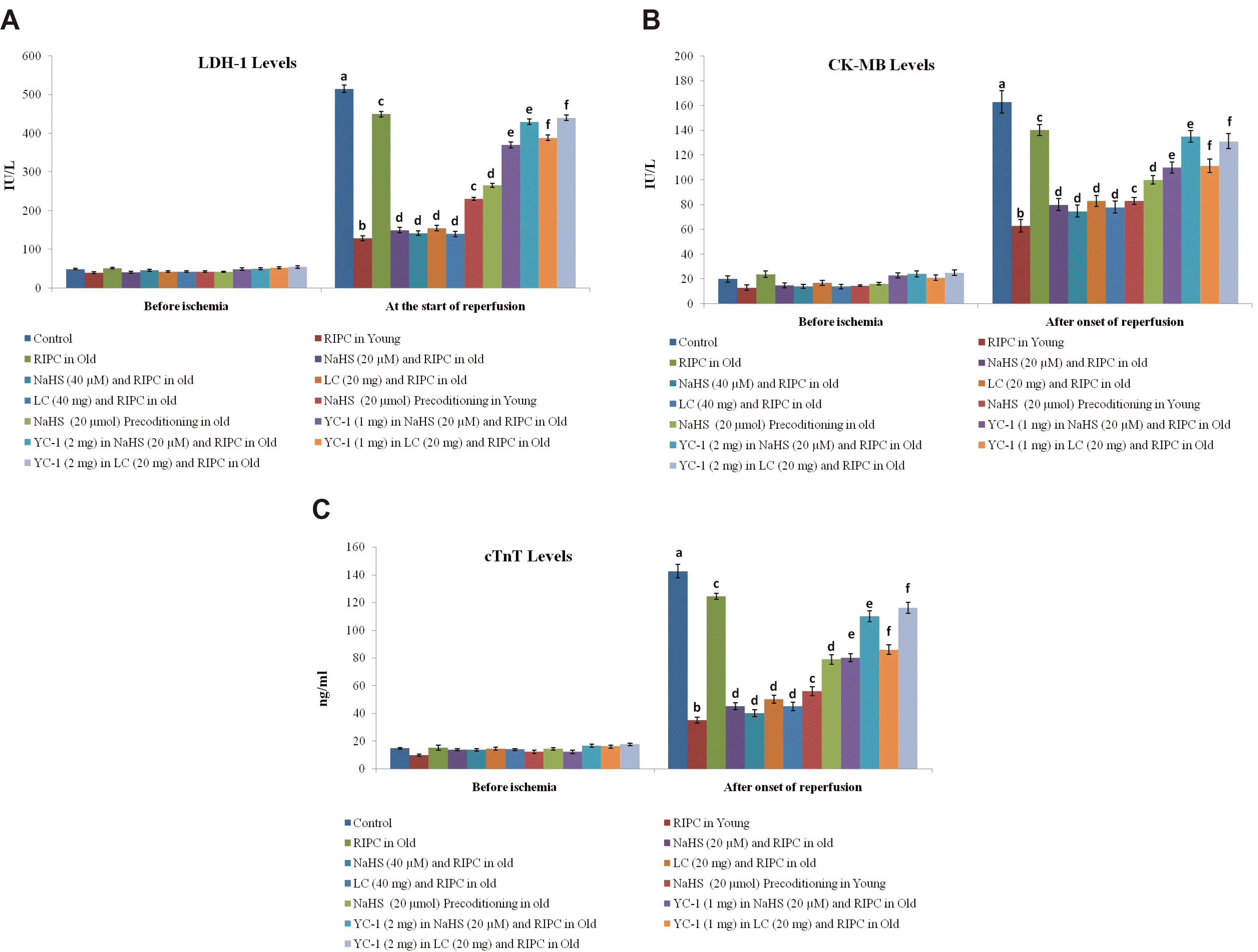
Fig. 2
Influence of remote ischemic preconditioning (RIPC), hydrogen sulfide (H2S) donor, H2S precursor and HIF-1α inhibitor on ischemia-reperfusion-induced myocardial infarction in young and old rats.
The values represent the percentage of infarcted area with respect to total area. HIF, hypoxia-inducible factor; NaHS, sodium hydrosulfide; LC, l-cysteine. ap < 0.05 vs. normal; bp < 0.05 vs. control; cp < 0.05 vs. RIPC in young; dp < 0.05 vs. RIPC in old; ep < 0.05 vs. NaHS (20 µM/kg) and RIPC in old; fp < 0.05 vs. LC (20 mg/kg) and RIPC in old.
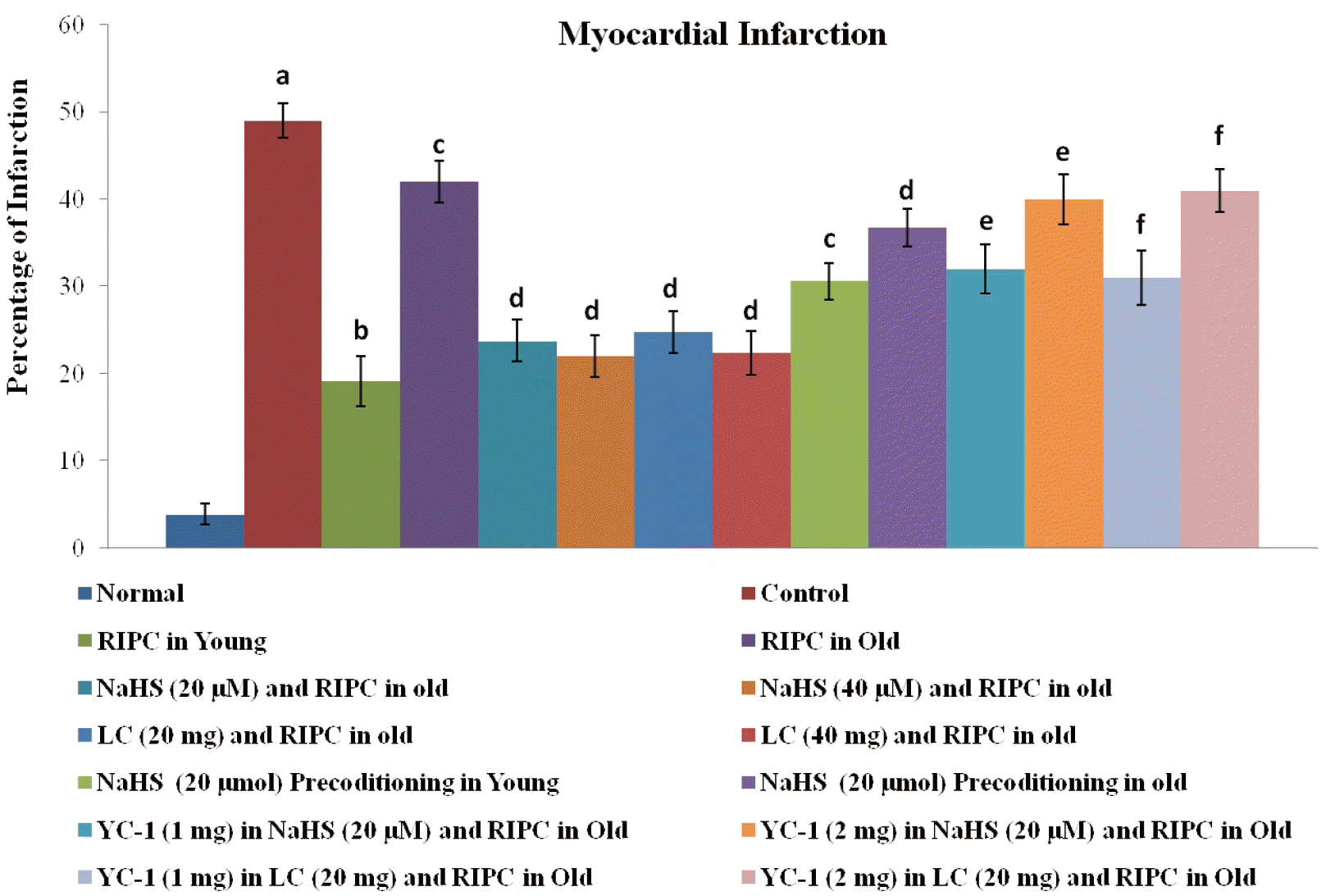
Fig. 3
Influence of remote ischemic preconditioning (RIPC), hydrogen sulfide (H2S) donor, H2S precursor and HIF-1α inhibitor on ischemia-reperfusion-induced changes in the expression of cystathionine β-synthase (A) and cystathionine γ-lyase (B) in the young and old rat hearts, after 120 min of reperfusion.
The values of normal groups were assigned 100% and values of all other groups were represented in the form of percentage of normal group. HIF, hypoxia-inducible factor; NaHS, sodium hydrosulfide; LC, l-cysteine. ap < 0.05 vs. normal; bp < 0.05 vs. control.
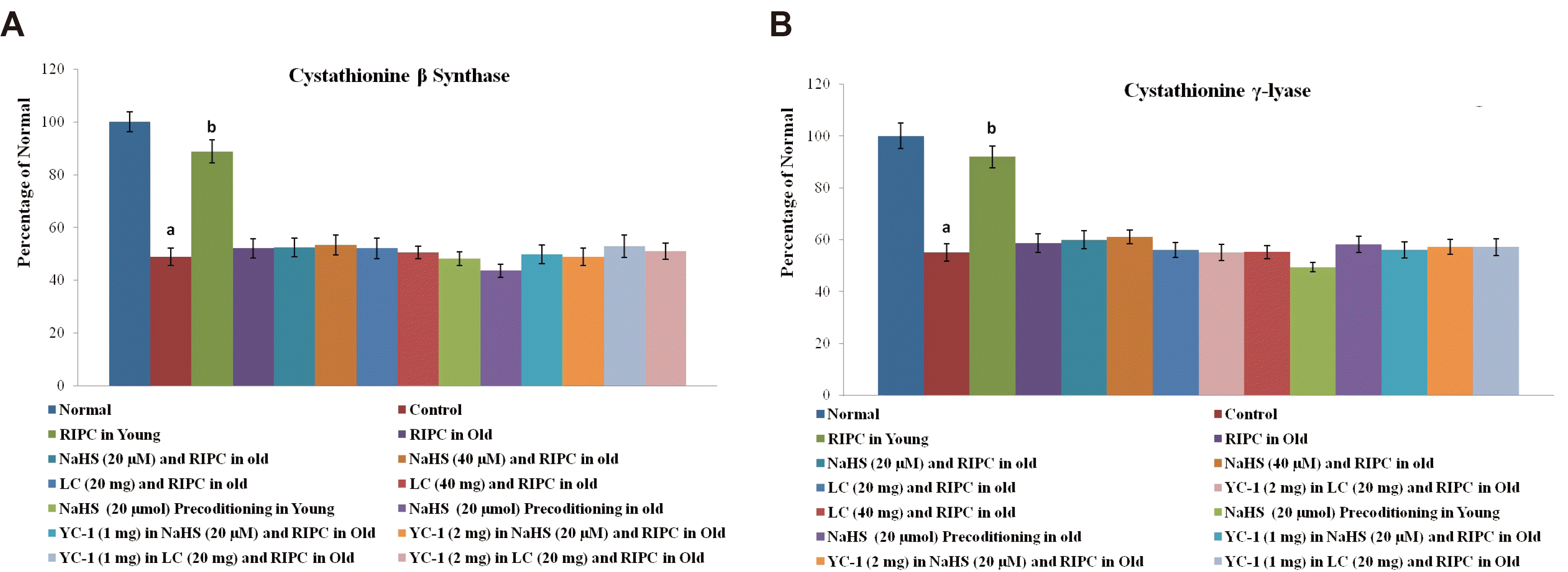
Fig. 4
Influence of remote ischemic preconditioning (RIPC), hydrogen sulfide (H2S) donor, H2S precursor and HIF-1α inhibitor on ischemia-reperfusion-induced changes in the H2S levels in young and old rat hearts, after 120 min of reperfusion.
HIF, hypoxia-inducible factor; NaHS, sodium hydrosulfide; LC, l-cysteine. ap < 0.05 vs. normal; bp < 0.05 vs. control; cp < 0.05 vs. RIPC in young; dp < 0.05 vs. RIPC in old; ep < 0.05 vs. NaHS (20 µM/kg) and RIPC in old; fp < 0.05 vs. LC (20 mg/kg) and RIPC in old.
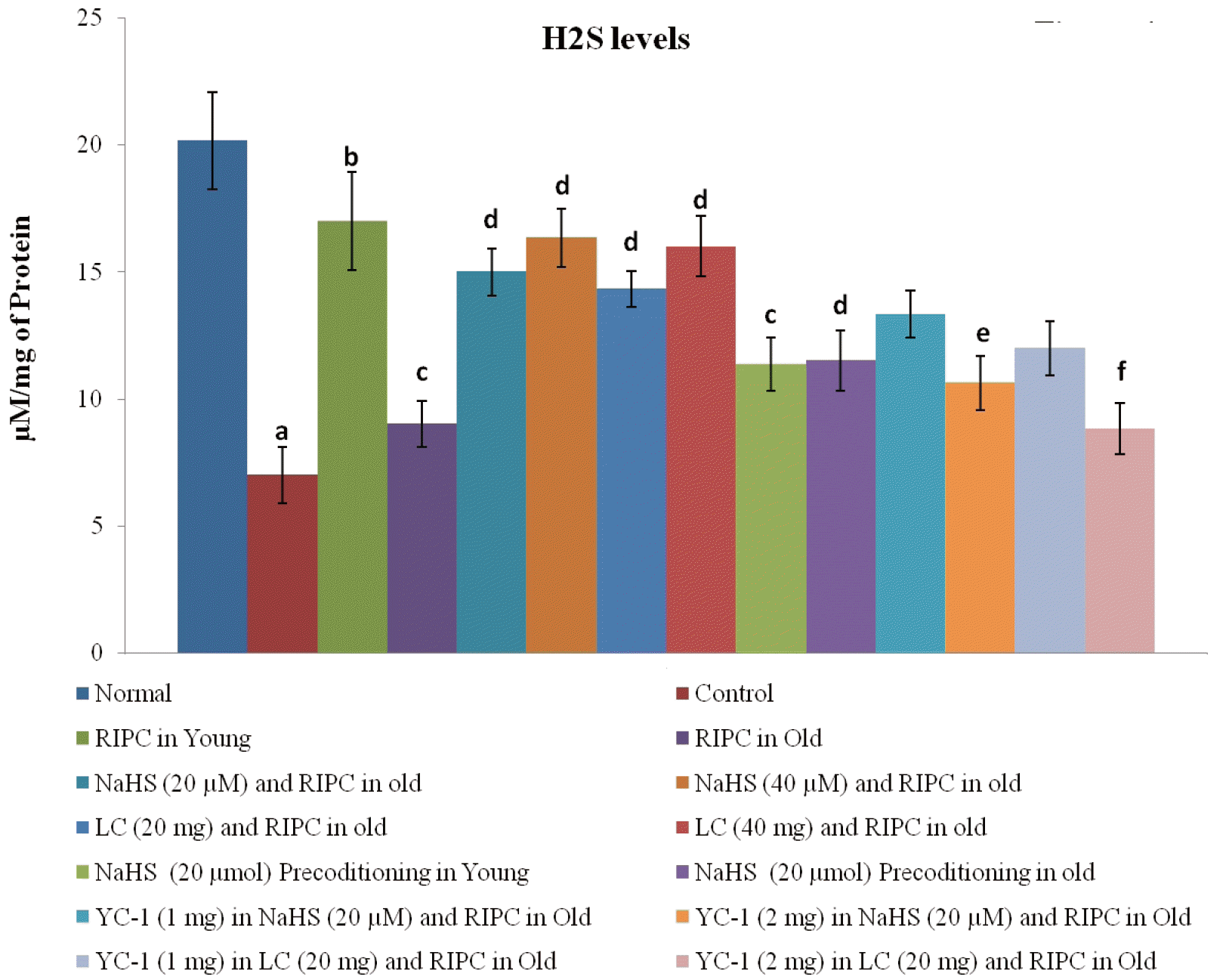
Fig. 5
Influence of remote ischemic preconditioning (RIPC), hydrogen sulfide (H2S) donor, H2S precursor and HIF-1α inhibitor on ischemia-reperfusion-induced changes in the nuclear: cytosolic ratio (in percentage) of Nrf2 (A) and HIF-1α levels (B) in young and old rat hearts, after 120 min of reperfusion.
The values of normal groups were assigned 100% and values of all other groups were represented in the form of percentage of normal group. HIF, hypoxia-inducible factor; Nrf2, nuclear factor erythroid 2-related factor 2; NaHS, sodium hydrosulfide; LC, l-cysteine. (A) ap < 0.05 vs. control; bp < 0.05 vs. RIPC in young; cp < 0.05 vs. RIPC in old; dp < 0.05 vs. NaHS (20 µM/kg) and RIPC in old; ep < 0.05 vs. LC (20 mg/kg) and RIPC in old. (B) ap < 0.05 vs. normal; bp < 0.05 vs. control; cp < 0.05 vs. RIPC in young; dp < 0.05 vs. RIPC in old; ep < 0.05 vs. NaHS (20 µM/kg) and RIPC in old; fp < 0.05 vs. LC (20 mg/kg) and RIPC in old.
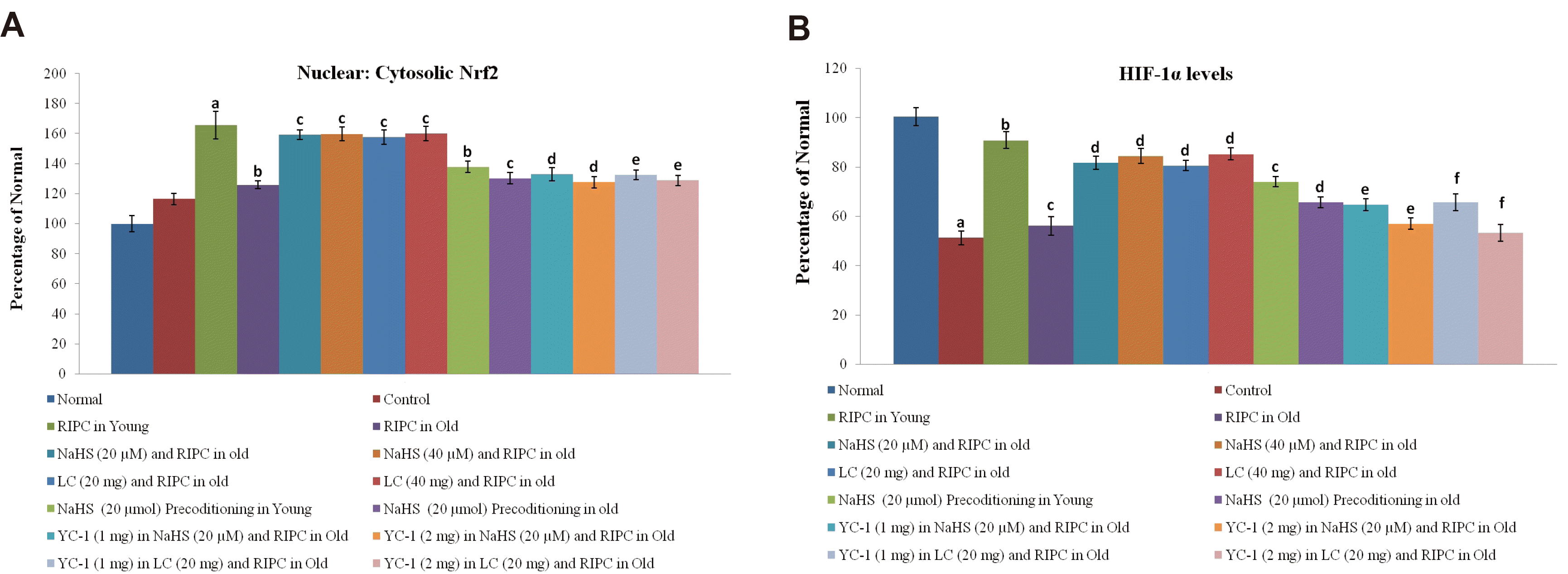
Fig. 6
Representative pictures of TTC-stained heart slices showing myocardial infarction in normal, control, RIPC in young and RIPC in old groups.
TTC, triphenyl tetrazolium chloride; RIPC, remote ischemic preconditioning.
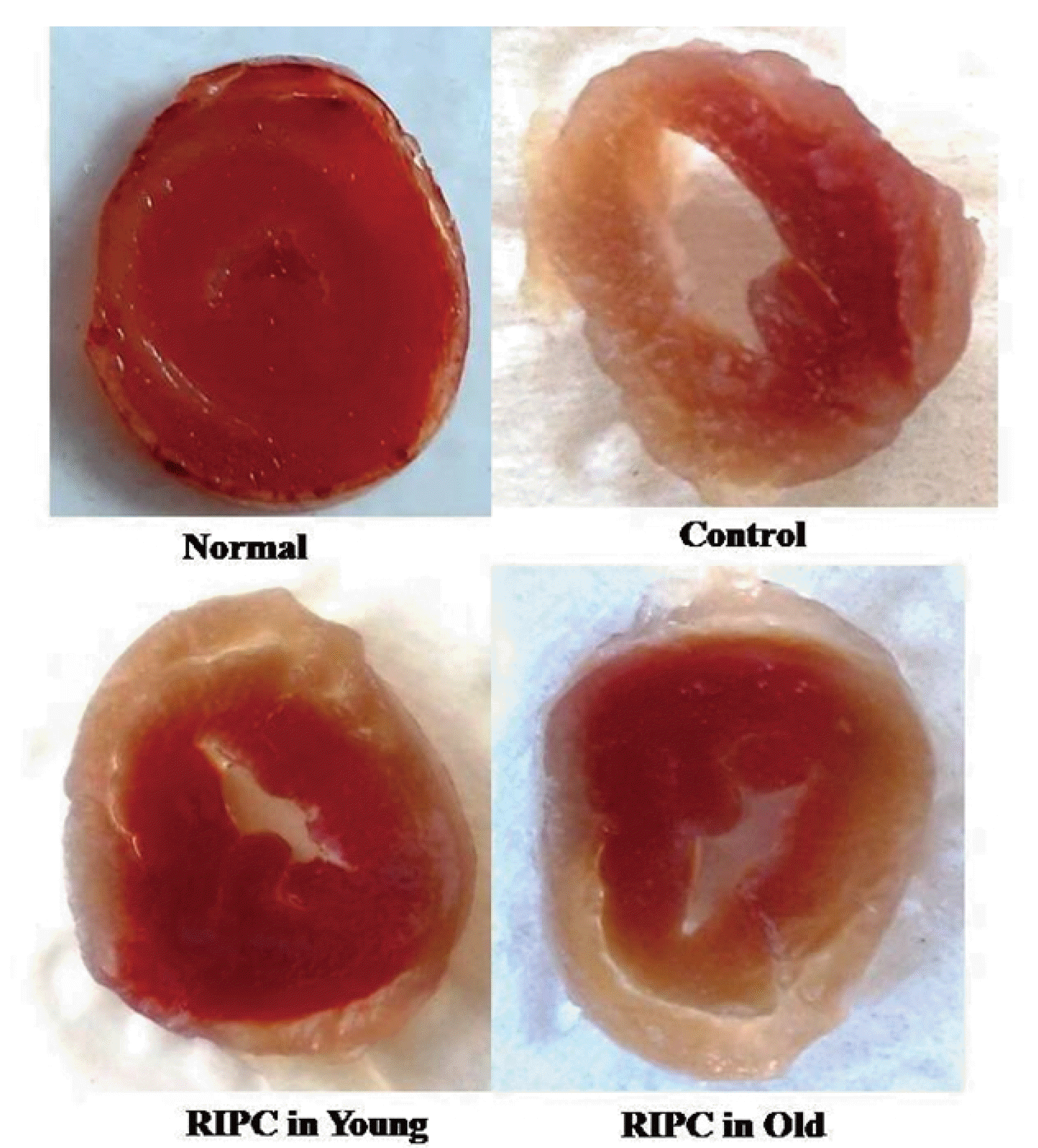
Table 1
Experimental protocol employed in the present study




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download