CASE REPORT
A 61-year-old female patient was admitted on June 12, 2019 for administration of the 5th cycle of chemotherapy for cholangiocarcinoma. The patient had been treated with cisplatin and gemcitabine from February 2019 to May 2019. At the time of admission, investigations showed white blood cell count 9.44×109/L, hemoglobin 8.1 g/dL, and platelet count 96×109/L. Lactate dehydrogenase (LDH) level was above normal (961 IU/L, reference range 273-490). Due to the low level of hemoglobin, one unit of packed red blood cells (RBCs) was requested.
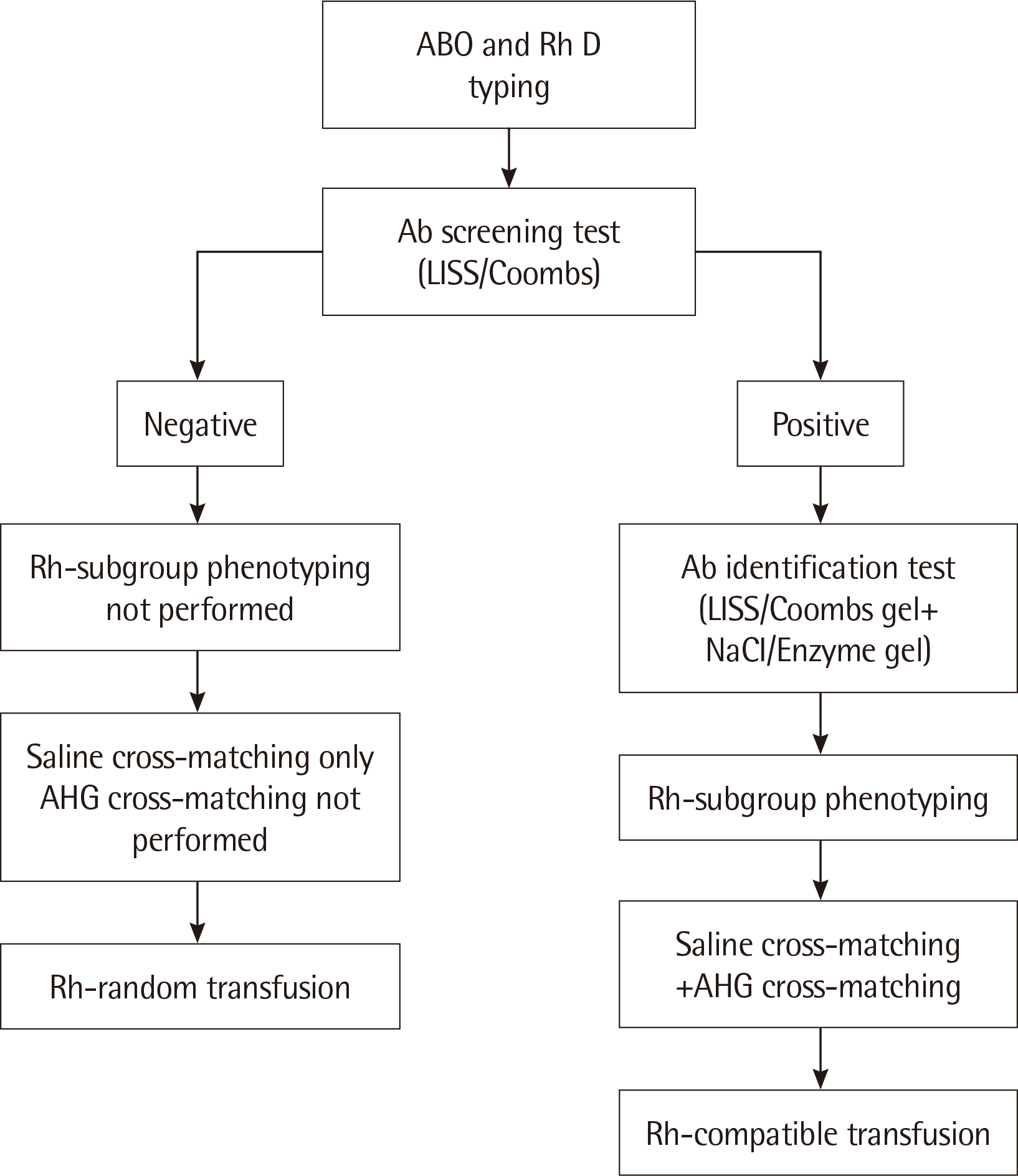
Pre-transfusion testing showed that the blood group of the patient was A and RhD positive. The patient’s blood sample was screened for unexpected antibodies using a 3-cell panel (0.8% Surgiscreen RBCs reagents 1, 2, and 3 [Ortho-Clinical Diagnostics Inc., Raritan, NJ, USA]) in the low-ionic-strength saline (LISS)/Coombs test. The antibody screening did not reveal any unexpected antibodies; therefore, Rh-subgroup typing for C, c, E, and e antigens was not performed (
Fig. 1). According to our pre-transfusion protocol, the anti-human globulin (AHG) phase of cross-matching is not required in cases that are negative for antibodies. Saline cross-matching showed compatibility between the donor RBCs and the recipient serum. Thus, the patient received Rh-random transfusion, as Rh-subgroup phenotyping was not performed. During transfusion, there were no symptoms or signs of adverse transfusion complications. Consequently, cisplatin was infused to the patient for 30 minutes. There was no complication during the administration of chemotherapy. However, one hour and 30 minutes after completion of the transfusion, and 40 minutes after infusion of the drug, the patient complained of diaphoresis, flank pain, and hematuria. A hemolytic transfusion reaction (HTR) was suspected, and consequently the patient was administered large amounts of fluids.
 | Fig. 1
The pre-transfusion protocol followed in our hospital according to the results of antibody screening.
Abbreviations: Ab, antibody; AHG, anti-human globulin; LISS, low-ionic-strength saline.

|
A transfusion reaction workup was initiated. LDH and serum bilirubin levels were found to be increased from 961 IU/L to 1870 IU/L and 0.7 mg/dL to 2.7 mg/dL, respectively. Additionally, significantly high level of cell-free plasma hemoglobin was observed (54.8 mg/dL, reference range <11). Peripheral blood smear revealed the presence of spherocytes without schistocytes. The direct antiglobulin test using polyspecific AHG (polyclonal anti-IgG and monoclonal anti-C3d) from Bio-Rad (Cressier-sur-Morat, Switzerland) was positive (1+). These findings and the clinical symptoms were suggestive of HTR.
To determine the causes of this adverse reaction, we performed additional tests using the pre-transfusion blood sample of the patient. Rh subgroup typing was performed, which revealed the presence of cE antigen on the RBCs of the patient. A repeat antibody screening using LISS/Coombs test from Ortho Clinical Diagnostics showed no unexpected antibodies, as observed previously. Another antibody screening using LISS/Coombs gel assay from Bio-Rad also did not reveal any unexpected antibodies. However, the NaCl/Enzyme gel assay revealed the presence of unexpected antibodies. Consequently, antibody identification using ID DiaPanel (Bio-Rad) was performed. 2+ reaction with rr indicated the presence of anti-e. 4+ and 3+ reactions were obtained with R1R1 and r’r, respectively, which indicated the presence of either anti-C, anti-Ce, or both along with anti-e (
Table 1). Saline cross-matching again showed compatibility between the donor RBCs and the recipient serum; however, AHG cross-matching using a LISS/Coombs gel card (Bio-Rad, Cressier, Switzerland) revealed incompatibility. Since anti-Rh antibodies were present in the blood of the patient, the previous transfusion history was reviewed for Rh-compatibility (
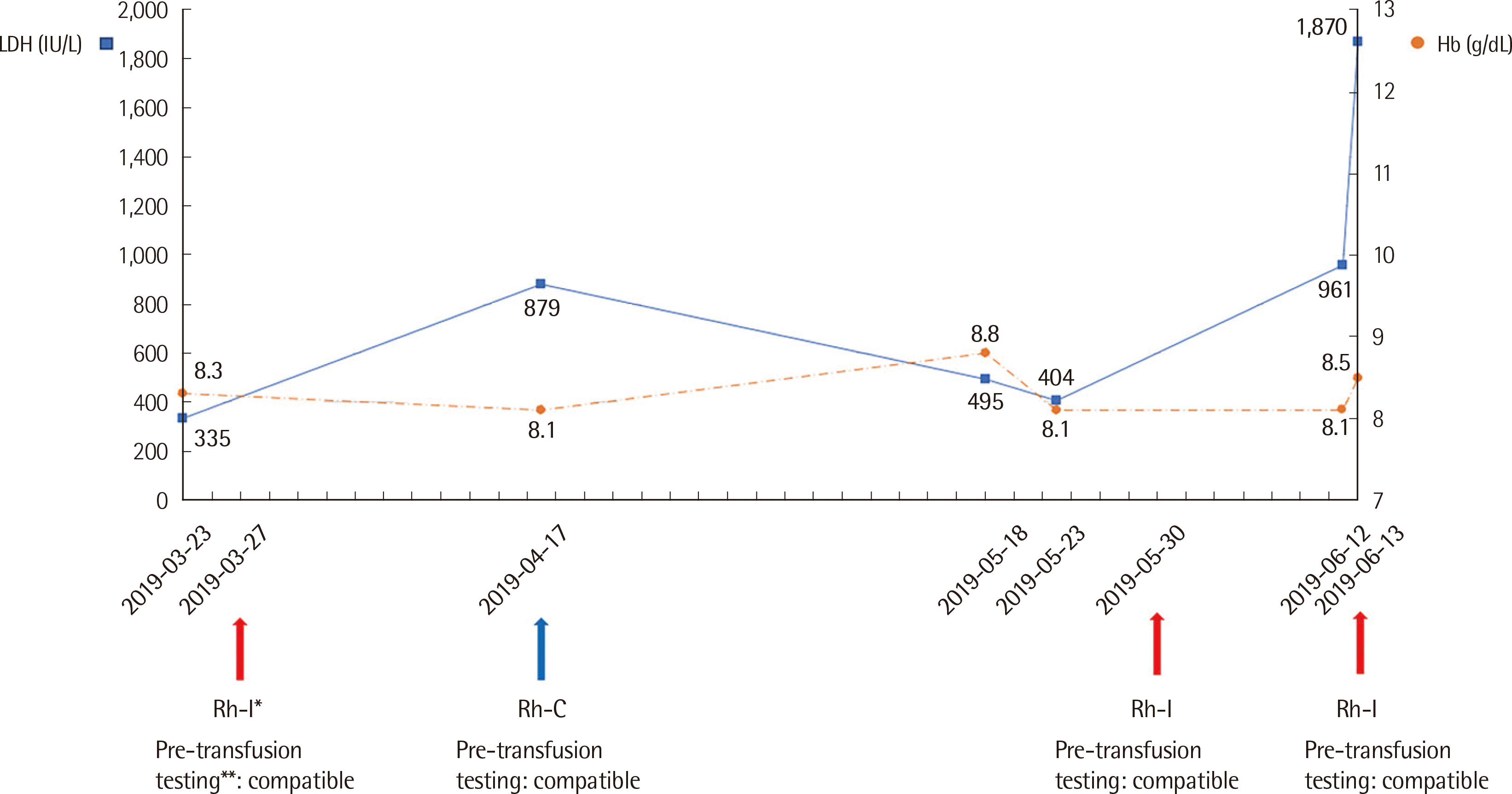
Fig. 2). Rh-incompatible transfusions had been administered thrice before, which led to the production of anti-Rh antibodies in the patient.
 | Fig. 2
Simplified laboratory values and history of multiple transfusions including antibody screening and cross-matching of the patient. *The donor blood used in every Rh-incompatible transfusion revealed the presence of C and e antigen. **Compatible pre-transfusion testing indicates negative antibody screening and compatible saline cross-matching.
Abbreviations: LDH, lactate dehydrogenase; Hb, hemoglobin; Rh-I, Rh-incompatible transfusion; Rh-C, Rh-compatible transfusion.

|
Table 1
Reactivity of the patient serum against representative reagent red cells
|
Rh-hr |
Rh genotype |
LISS/Coombs |
Enzyme |
|
CCD.ee |
R1R1 |
+/- |
4+ |
|
ccD.EE |
R2R2 |
+/- |
- |
|
Ccddee |
r’r |
+/- |
3+ |
|
ccddee |
rr |
+/- |
2+ |
|
Auto control |
|
+/- |
- |

Diaphoresis disappeared a few hours after hydration therapy. Hematuria and flank pain resolved on the 6th day after transfusion, and the patient was discharged from the hospital. One month after discharge, the patient was admitted again and received Rh-compatible transfusion, followed by administration of cisplatin, and showed no signs and symptoms of acute hemolysis: LDH level did not increase significantly (from 410 IU/L to 442 IU/L) and serum bilirubin remained unchanged at 0.5 mg/dL. The patient did not complain of diaphoresis, flank pain, or hematuria.
Go to :

DISCUSSION
Hemolytic transfusion reactions can be divided into acute and delayed hemolytic transfusion reactions [
1-
3]. Acute hemolytic transfusion (AHTR) reaction occurs during or within 24 hours of completion of the transfusion, whereas delayed hemolytic transfusion reaction (DHTR) occurs between 1 day and 28 days after completion of transfusion [
1-
3]. Most of the immune-mediated alloantibodies against Rh blood group system induce predominantly extravascular hemolysis and DHTR [
1,
2]. AHTR typically occurs when preformed IgM ABO antibodies attack the incompatible transfused red blood cells by complement fixation, leading to intravascular hemolysis [
1,
2]. However, intravascular hemolysis and AHTR can also be triggered by non-ABO antibodies [
4,
5]. Although there is no consensus on whether Rh antibodies can induce AHTR and intravascular hemolysis, sporadic cases of AHTR due to Rh antibodies have been previously reported [
4,
6-
11]. Rh system is the most complex among all blood group systems, and more than 49 antigens have been identified [
12]. The five important Rh antigens are D, c, E, C, and e in the order of decreasing immunogenicity [
4]. The Rh phenotype of the patient, in the present study, was RhDcE. The Rh phenotype of two of the three donor RBCs previously transfused to the patient was RhDCe (
Fig. 1). Due to these multiple Rh-incompatible transfusions, the patient was alloimmunized to C and e antigens, and produced the corresponding antibodies, which remained undetected during our pre-transfusion testing. In this patient, symptoms of hematuria and flank pain were observed along with elevated LDH, bilirubin, hemoglobinemia, and spherocytes on blood smear, all of which occurred within 24 hours of completion of transfusion. In addition, direct anti-globulin test was positive (1+). Antibody identification results demonstrated the presence of anti-e (RH5) with either anti-C (RH2), anti-Ce (RH7), or both (Rh2+Rh7) (
Table 1). These findings suggest AHTR due to combined Rh antibodies against C and e antigens. However, serologic evidences alone do not meet the “definite” criteria for AHTR according to the CDC diagnostic criteria; an elution test is necessary for confirmation of the diagnosis [
3]. Hence, the diagnosis of our case is “probable” AHTR. Although cases of AHTR due to either only anti-C (RH2) [
11] or only anti-e (RH5) [
10] have been reported, to the best of our knowledge, AHTR due to alloantibodies against both C and e antigens has never been previously reported. Clinical impact caused by alloantibodies against both C and e antigens includes DHTR [
11] and hemolytic disease of the newborn [
13-
17]. The impact of alloantibodies against C and e antigens has limited clinical significance, possibly due to the low immunogenicity of these antigens [
18]. However, the present case shows a very rare significant clinical impact of the alloantibodies against C and e antigens.
The pre-transfusion protocol at our institution failed to detect the unexpected antibodies produced by multiple previous Rh-incompatible transfusions (
Fig. 2). The culprit antibody was only detected by antibody screening and antibody identification using NaCl/Enzyme gel cards (Bio-Rad), retrospectively. This might be because the enzyme method could enhance the detection of Rh antibody [
19]. In this patient, the early onset of HTR observed after negative screening for antibodies is unusual. The reason for this rapid hemolysis caused by the alloantibodies is difficult to elucidate [
20]. In this context, it is pertinent to mention that AHTR due to Rh antibody might occur without detectable alloantibodies [
6-
9]. The present case adds to the evidence that ‘in vitro’ tests for detection of alloantibodies might not always predict the extent, if any, of RBC hemolysis ‘in vivo’ [
11]. Despite the uncertainty, the negative result of antibody screening with the LISS/Coombs method requires further explanation. The likelihood of production of RBC antibodies by the recipient’s immune system depends on various factors such as innate and acquired patient factors, the number of transfusions, and the immunogenicity of the RBC antigens [
18]. Our patient suffered from cholangiocarcinoma and was consequently under chemotherapy (cisplatin). Cancer is known to induce immunosuppression [
21], and cisplatin might cause bone marrow suppression [
22]. Thus, it is possible that the capacity for antibody production was reduced in this patient, which might have led to low titers of the alloimmunized antibodies. In addition to this, as previously stated, the C and e antigens have low immunogenicity; the inherent nature of these antigens might have played a role in reducing the potency or titer of the corresponding antibodies. Unfortunately, since antibody titration was not performed and the immunoglobulin levels were not available, we were unable to test the hypotheses for the negative antibody screening. Further research to understand the antibody reaction discrepancy between the ‘in vitro’ and ‘in vivo’ is necessary.
AHTR symptoms manifested 40 minutes after completion of the 5th cycle of cisplatin. Cisplatin is one of the most widely used anti-cancer drugs for solid tumors [
22]. Anemia is thought to occur due to myelosuppression of the bone marrow by cisplatin [
22]. Very rarely, acute hemolysis has been reported in a patient with a history of repeated exposure to cisplatin [
23]. Thus, given the timing of the reaction and the known complication of cisplatin, it is possible that cisplatin-induced hemolysis might have occurred in our patient. Due to the lack of an elution test, as noted previously, we could not completely rule out the possibility of acute hemolysis caused by cisplatin. This is the limitation of our study. However, we believe, the fact that the patient subsequently received Rh-compatible transfusion and cisplatin, and did not develop hemolysis, excludes the possibility of cisplatin-induced hemolysis.
The preformed Rh antibodies against C and e antigens in the present case were detected retrospectively using the enzyme method for antibody screening and identification, and AHG cross-matching. Subsequent Rh-compatible transfusion after full Rh-phenotyping prevented further adverse complications. These findings indicate that the current pre-transfusion protocol might miss a clinically significant alloantibody. Recipient clinical factors are known to iñuence the risk of RBC alloimmunization [
24]. An increased risk of alloimmunization is associated with solid malignancies [
25], the number of units transfused [
24], hematopoietic stem cell transplantation [
25], myelodysplastic syndrome [
26], or hemoglobinopathy [
26]. In this case, the patient suffered from solid tumor and received multiple transfusions, which are known risk factors for RBC alloimmunization. Additional pre-transfusion testing such as extended RBC phenotype can considerably reduce the risk of RBC alloimmunization in patients with hemoglobinopathy, undergoing chronic transfusion [
27,
28]. Previous studies suggested that extended RBC phenotype testing might be necessary for patients with myelodysplastic syndrome who require chronic transfusion [
29]. Some blood bank laboratories perform AHG cross-matching regardless of the transfusion history or antibody screening result [
30]. Likewise, as the present case suggests, additional pre-transfusion testing might be beneficial in patients who have known risk factors for RBC alloimmunization, to avoid possible adverse transfusion complications. Therefore, blood bank laboratories should thoroughly assess the risk factors for RBC alloimmunization in the recipients and perform pre-transfusion testing accordingly. Additionally, clinicians should be aware of the possibility of adverse transfusion reaction in a patient despite negative antibody screening and compatible cross-matching.
In summary, the present case highlights that AHTR due to anti-Rh antibodies against C and e antigens might occur without detectable antibodies. Caution should always be exercised during or after transfusion to monitor possible complications in a patient, for timely diagnosis and proper management.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download