Abstract
Parent-to-child transmission of ring chromosome (RC) 21 with retained mosaicism has been reported very rarely. Here, we report a case of familial RC 21 mosaicism composed of several derivative clones resulted from mitotic instability of RC 21. Mother had mos 46,XX,r(21)(p13q22.3)[12]/46,XX[8], and her daughter had a more complex mosaicism, mos 47,XX,+r(21)(p13q22.3)mat[4]/45,XX,-21[3]/46,XX,r(21)(p13q22.3)mat[1]/47,XX,+dic r(21)[1]/46,XX[22].
Go to : 
초록
부모에게서 자손으로 유전되는 ring chromosome (RC) 21 모자이크현상은 매우 드물게 보고되어 있다. 저자들은 RC 21의 유사분열 불안정성으로 인해 초래된 여러 개의 다른 클론으로 구성된 가족성 RC 21 모자이크현상을 보고하고자 한다. 어머니의 핵형은 mos 46,XX,r(21)(p13q22.3)[12]/46,XX[8]이었으며, 딸의 핵형은 좀 더 복잡한 모자이크현상을 보여 mos 47,XX,+r(21)(p13q22.3)mat[4]/45,XX,-21[3]/46,XX,r(21)(p13q22.3)mat[1]/47,XX,+dic r(21)[1]/46,XX[22]이었다.
Go to : 
Ring chromosome (RC) is a circular DNA that results from breaks at the ends of both the chromosome arms followed by fusion of the broken ends [1]. During ring formation, genetic material might be lost or a complete ring with no deletion can be created by fusion of subtelomeric sequences or telomere–telomere fusion [2]. Most RCs are formed de novo. Only about 1% of RCs have been found to be inherited from parents, and the most common RCs are RC 20, 21, 22, and 14 [3-5]. The reasons for the rare inheritance of RCs are thought to be the instability of the ring during meiosis and infertility of the carriers [3]. Among the inherited RC 21, cases of familial mosaicism in which mosaicism is retained between the generations have been found very rarely [6, 7].
Here, we report a rare case of familial RC 21 mosaicism where mother had mos 46,XX,r(21)(p13q22.3)[12]/46,XX[8]. Her daughter inherited RC 21 from her, but had a more complex mosaicism, mos 47,XX,+r(21)(p13q22.3)mat[4]/45,XX,-21[3]/46,XX,r(21)(p13q22.3)mat[1]/47,XX,+dic r(21)[1]/46,XX[22].
Go to : 
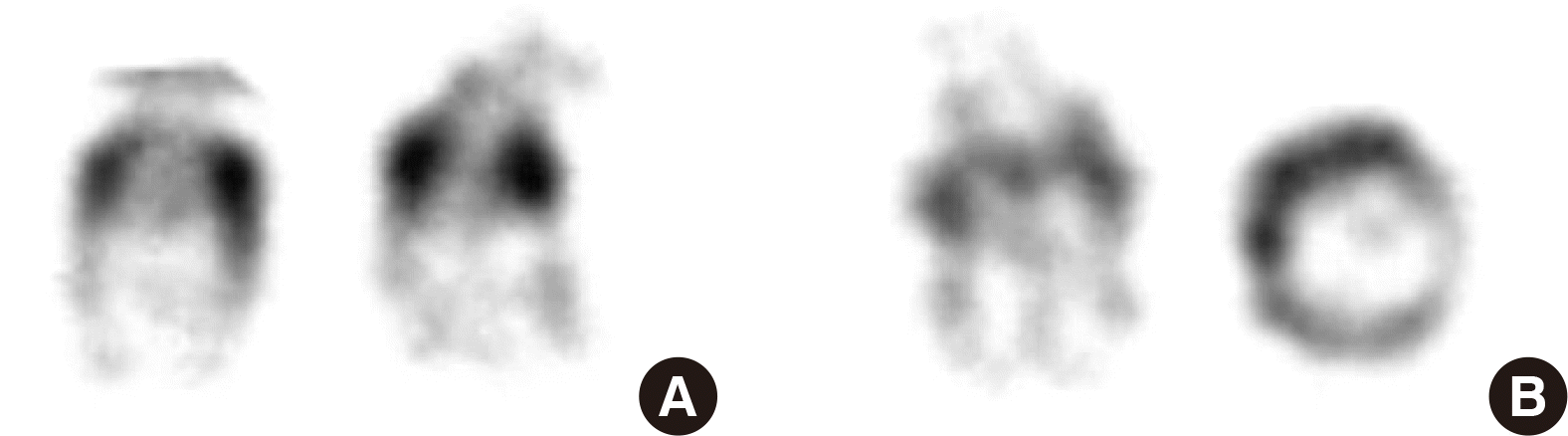
The proband, a 2-month-old girl, was the second-born child of unrelated parents. Her parents and 5-year-old sister had normal phenotype and intelligence. Her mother, aged 38, without any dysmorphism, had a history of spontaneous abortion (4 times) and a heart surgery due to atrial septal defect (ASD). To find out the reason for recurrent abortions, karyotyping was performed, which revealed mos 46,XX,r(21)(p13q22.3)[12]/46,XX[8] (Fig. 1). Microarray analysis using a CytoScan 750K array (Affymetrix, Santa Clara, CA, USA) detected no loss or gain of genetic material.
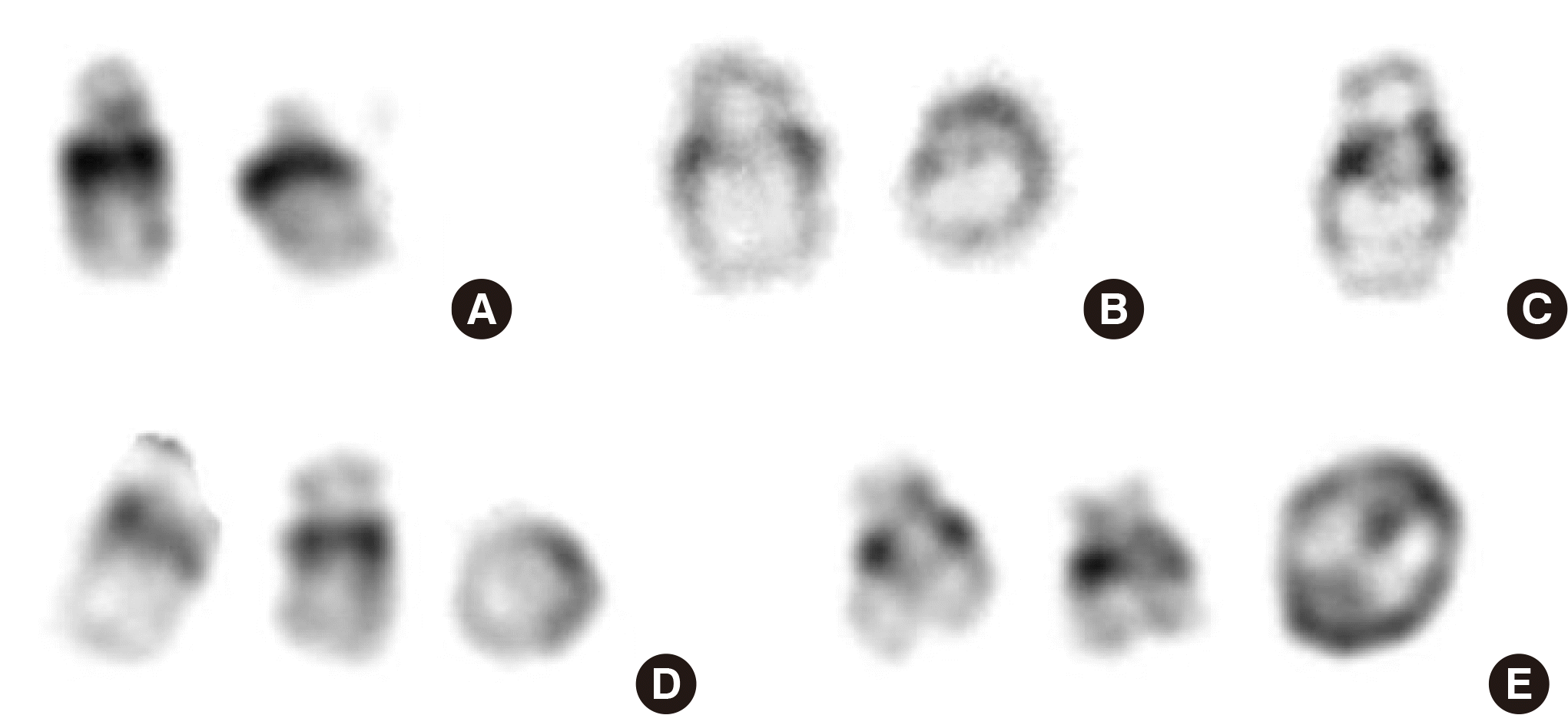
The proband was born at 38 weeks of gestation by caesarean section, with birth weight of 3,200 g. Karyotype by amniocentesis was normal at 16 weeks of gestation. However, after birth, low nasal bridge, mild facial dysmorphism, hypotonia, and a 2.3 mm ASD were detected. Conventional karyotyping revealed mos 47,XX,+r(21)(p13q22.3)mat[4]/45,XX,-21[3]/46,XX,r(21)(p13q22.3)mat[1]/47,XX,+dic r(21)[1]/46,XX[22] (Fig. 2). Re-analysis of the same amniotic fluid sample revealed very low-level mosaicism of RC 21 (8 of the 100 metaphase cells examined).
Fluorescence in situ hybridization using RUNX1(21q22) probes showed three and one RUNX1 signals in 16% and 5% of the 200 metaphase cells, respectively. Microarray analysis for proband and karyotyping for proband’s father and older sister were recommended through genetic counselling; however, those tests were refused.
Go to : 
Inheritance of RC is a very rare phenomenon. RC 22, RC 21, RC 20, and RC 14 are the most common among inherited RCs [3, 5]. This preference might be because of their relative stability during cell division and less phenotypic effect of these small chromosomes than the larger ones [3]. Particularly, high rate of mother-to-child RC transmission than father-to-child has been observed, although there are no sex differences in the prevalence of RC in general [3, 8]. This difference is thought to be due to the deleterious effect of RC on spermatogenesis in male. However, no distinct effect on fertility has been reported in female [9].
In a review article, 9 out of 30 families with ring transmission showed that both the parent and the child were mosaic [3]. In our case of RC 21, both the mother and the daughter showed mosaicism. As the germline transmission of an RC should produce a non-mosaic karyotype, the reproduction of mosaicism in the offspring should be rare. Several hypotheses have been proposed to explain the co-occurrence of normal karyotype with RC in the offspring who has mosaic RC carrier parents: 1) early postzygotic loss of the ring and subsequent reduplication (monosomy rescue), 2) ring opening during early embryogenesis, and 3) postzygotic ring re-formation [3, 10, 11]. However, in addition to normal clone, our patient had several derivative clones, which could be explained by “dynamic mosaicism” [12, 13]. During cell division, if a ring replicates without sister chromatid exchange in the prophase, the separation of the ring results in the equal-sized rings. If sister chromatid exchange occurs through dicentric ring or interlocked ring formation, followed by asymmetric breakage, variable unbalanced products such as ring loss, dicentric RC, and duplication or additional RC could be produced in the daughter cells. This continuous production of aberrations in the daughter cells is referred as “dynamic mosaicism” [12-14].
Generally, inherited RC shows milder symptoms than sporadic case, which is more striking in the familial RC cases that rarely show major malformation [6]. However, formation of an additional RC 21 or duplication of Down syndrome critical region by dynamic mosaicism can lead to Down syndrome in RC 21 carriers [15, 16]. In a report, the authors reviewed 26 cases of RC 21 carriers and found that 5 (21%) of them had Down syndrome offspring, whose karyotype were either duplication of RC 21 or additional RC 21 [6]. Our patient had additional RC 21 (16% by fluorescence in situ hybridization) and dicentric RC 21 clones, and showed low nasal bridge, mild facial dysmorphism, and hypotonia, which were non-specific to be diagnosed as Down syndrome. The absence of symptoms or the presence of mild symptoms might be due to a low fraction of +21 clone or different proportion of mosaicism in different organs. Although she had ASD, we cannot be sure if ASD is related to RC 21 or trisomy 21, because her mother also had the same ASD. Therefore, long-term follow-up for proband is needed for the identification of more specific Down syndrome-related symptoms.
Here, we report a very rare case of a familial RC 21 mosaicism. When a new case of RC 21 is detected, detailed karyotyping for many metaphase cells should be performed to detect the mosaicism with variable RC 21 derivative clones. In addition, we recommend genetic counseling and family study, including parental and prenatal tests, because of the possibility of inheritance and recurrence of RC.
Go to : 
REFERENCES
1. Sigurdardottir S, Goodman BK, Rutberg J, Thomas GH, Jabs EW, Geraghty MT. 1999; Clinical, cytogenetic, and fluorescence in situ hybridization findings in two cases of "complete ring" syndrome. Am J Med Genet. 87:384–90. DOI: 10.1002/(SICI)1096-8628(19991222)87:5<384::AID-AJMG3>3.0.CO;2-R.


2. Le Caignec C, Boceno M, Jacquemont S, Nguyen The Tich S, Rival JM, David A. 2004; Inherited ring chromosome 8 without loss of subtelomeric sequences. Ann Genet. 47:289–96. DOI: 10.1016/j.anngen.2003.10.005. PMID: 15337475.

3. Kosztolányi G, Méhes K, Hook EB. 1991; Inherited ring chromosomes: an analysis of published cases. Hum Genet. 87:320–4. DOI: 10.1007/BF00200912. PMID: 1864607.


4. Ikeuchi T, Yamamoto K, Qiao F, Hayakawa K, Migita T, Nishikawa Y. 1990; Ring chromosome 21 transmitted from mother to daughter: its stability in a lymphoblastoid cell line. Ann Genet. 33:32–5.

5. Arnedo N, Nogués C, Bosch M, Templado C. 2005; Mitotic and meiotic behaviour of a naturally transmitted ring Y chromosome: reproductive risk evaluation. Hum Reprod. 20:462–8. DOI: 10.1093/humrep/deh598. PMID: 15528264.


6. Bertini V, Valetto A, Uccelli A, Tarantino E, Simi P. 2008; Ring chromosome 21 and reproductive pattern: a familial case and review of the literature. Fertil Steril. 90:2004.e1–5. DOI: 10.1016/j.fertnstert.2008.01.087. PMID: 18371955.

7. Howell RT, McDermott A, Gardner A, Dickinson V. 1984; Down's syndrome with a recombinant tandem duplication of chromosome 21 derived from a maternal ring. J Med Genet. 21:310–4. DOI: 10.1136/jmg.21.4.310. PMID: 6238166. PMCID: PMC1049305.



8. MacDermot KD, Jack E, Cooke A, Turleau C, Lindenbaum RH, Pearson J, et al. 1990; Investigation of three patients with the "ring syndrome", including familial transmission of ring 5, and estimation of reproductive risks. Hum Genet. 85:516–20. DOI: 10.1007/BF00194228. PMID: 2227937.


9. Tulloch WS, Newsam JE. 1966; Studies on human meiotic chromosomes from testicular tissue. Lancet. 1:679–82. DOI: 10.1016/S0140-6736(66)91627-8.

10. Speevak MD, Smart C, Unwin L, Bell M, Farrell SA. 2003; Molecular characterization of an inherited ring (19) demonstrating ring opening. Am J Med Genet A. 121A:141–5. DOI: 10.1002/ajmg.a.20184. PMID: 12910493.


11. Surace C, Berardinelli F, Masotti A, Roberti MC, Da Sacco L, D'Elia G, et al. 2014; Telomere shortening and telomere position effect in mild ring 17 syndrome. Epigenetics Chromatin. 7:1. DOI: 10.1186/1756-8935-7-1. PMID: 24393457. PMCID: PMC3892072.

12. Pristyazhnyuk IE, Menzorov AG. 2018; Ring chromosomes: from formation to clinical potential. Protoplasma. 255:439–49. DOI: 10.1007/s00709-017-1165-1. PMID: 28894962.


14. McDermott A, Voyce MA, Romain D. 1977; Ring chromosome 4. J Med Genet. 14:228–32. DOI: 10.1136/jmg.14.3.228. PMID: 881718. PMCID: PMC1013566.



15. Matsubara T, Nakagome Y, Ogasawara N, Oka S, Yokochi T. 1982; Maternally transmitted extra ring (21) chromosome in a boy with Down's syndrome. Hum Genet. 60:78–9. DOI: 10.1007/BF00281270. PMID: 6210618.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download