Blood transfusion is useful for saving lives, but has inevitable side effects, such as adverse transfusion reactions (ATRs). ATR reporting and analysis are aimed at preventing ATRs. Although ABO-incompatible (ABOi) transfusion can lead to acute hemolytic transfusion reactions (AHTRs), one of the most serious ATRs, underreporting of these incidents is a concern. The frequency of ABOi transfusions is estimated to range from 1/10,000 to 1/4,667 blood units, based on the reporting of clinical episodes [
1,
2]. In Korea, hemovigilance has been practiced since 2010 [
3]. The frequency of ABOi transfusions in Korea was 1/1,390,378 red blood cell (RBC) units in 2017 and 1/1,446,265 RBC units in 2018 as reported in the Korean hemovigilance system (KOHEVIS) [
4]. Inconsistencies between the ABOi transfusion frequencies in the literature and the KOHEVIS can be attributed to underreporting. Therefore, we comprehensively analyzed ABOi transfusion events in Korea, including those from KOHEVIS annual reports (from 2008 to 2018), literature (from 1978 to 2019), and written judgments (from 1953 to 2019). This is the first study to analyze written judgments for ABOi transfusions. We considered ABOi RBC transfusions as well as unintended ABOi platelet and fresh frozen plasma (FFP) transfusions.
The Institutional Review Board of Samsung Medical Center, Seoul, Korea (file no. 2019-10-044) exempted the approval of this study.
The Court of Korea has provided a wide range of public judicial information services, including a comprehensive legal information system and an internet webpage (e-Court system) that allows case searches. Data on most criminal, civil, patent, and administrative cases filed after 1998 can be retrieved and assessed using a written judgment management system by entering the court name, case number, or search terms [
5]. Some trials before 1998 can be assessed through the comprehensive legal information system [
6].
In this study, the term “transfusion” was searched, and the cases found were included based on different factors specific to the search results. Cases were excluded when a written judgment was not available, when “transfusion” was referred to as “capital injection,” or when transfusion was considered to be a subject of dispute. Only ABOi transfusion cases were included in this study. Data reported to KOHEVIS were collected (
http://www.kohevis.or.kr). ABOi transfusion cases reported in the Korean literature were obtained through searches of KoreaMed (Korean Association of Medical Journal Editors, Seoul, Korea) and PubMed (US National Library of Medicine, Bethesda, MD, USA).
In total, 756 cases were found using the search term “transfusion.” Of these, 186 cases were excluded; 173 were related to non-medical issues, eight were duplicated, and five included inaccessible written judgments. Of the 570 written judgments that were reviewed, only 152 cases included “transfusion” as the main subject. Among these, 140 cases were classified according to different topics, such as transfusion indication, blood product preparation, infectious transfusion reactions, other ATRs, and refused transfusion and hence excluded. Twelve cases were classified as cases of ABOi transfusion; however, three of these did not contain definite ABO typing information. Finally, nine cases of ABOi transfusion (cases 1 to 9) were included in this study (
Fig. 1). There were seven cases from criminal trials (cases 2–7, and 9) and two from civil trials (cases 1 and 8). The defendants were doctors in seven cases (cases 1, 2, 4, and 6–9), nurses in six cases (cases 2–5, 7, and 9), and a medical laboratory technologist in one case (case 6). There were one testing error (ABO typing using a sample from another patient in the same laboratory, case 6), one sampling error (ABO typing using another patient’s sample, case 8) and administration error (transfusions given to unintended patients caused by misidentification between blood bags and patients, case 1–5, 6, and 9). ABOi RBCs and ABOi FFP were transfused in five and two cases, respectively. In the other two cases, ABOi blood was transfused, but the product type was unknown. The average age of the cases was 50.4 years (the age in one case was not described in the judgment), and there were seven male and two female cases.
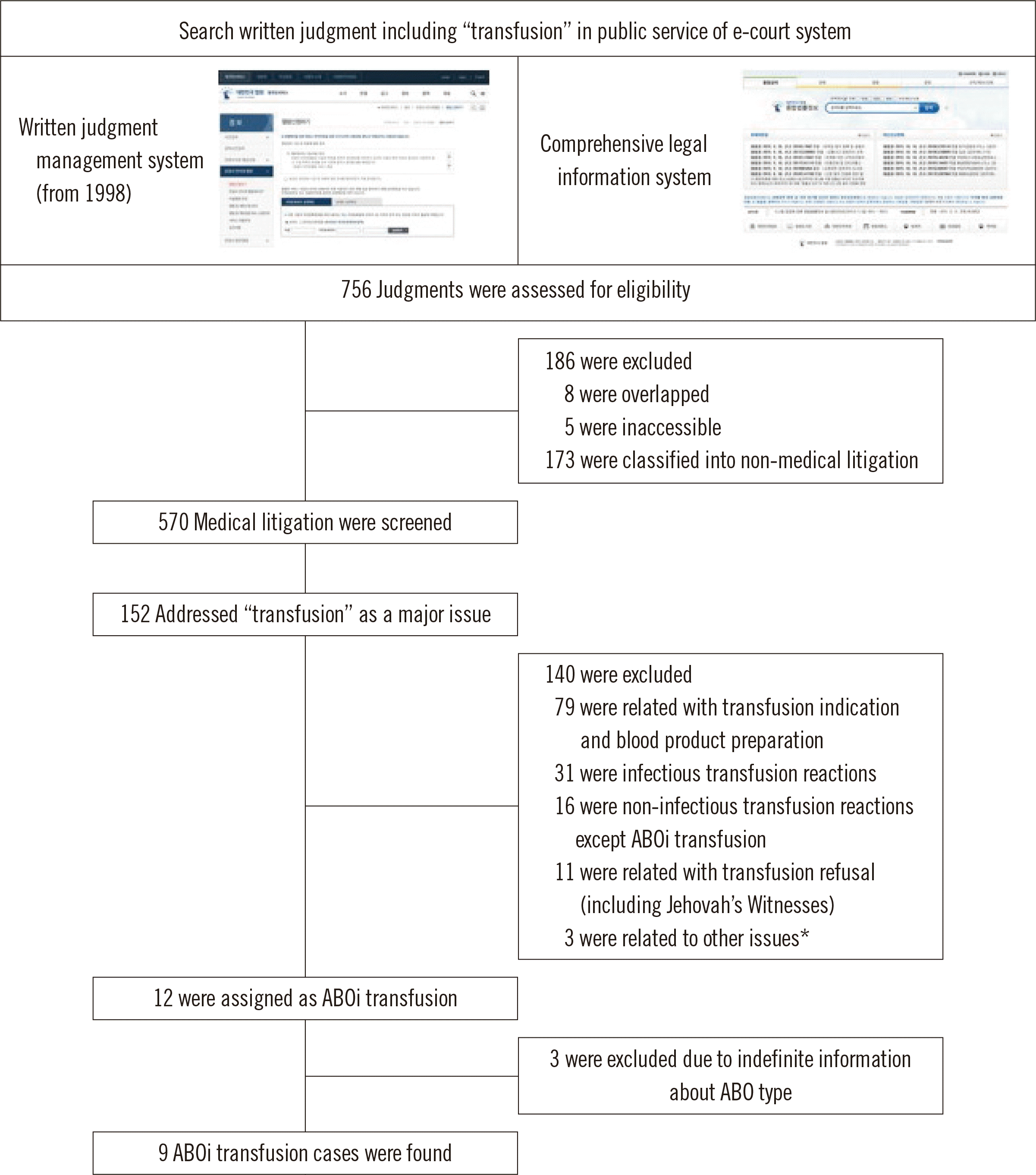 | Fig. 1
Flowchart of the inclusion of data from written judgments.
*In one case, the patient expired because the needle of transfusion set was removed and the blood was not infused. In two cases, there were incorrectly recorded transfusion time in the medical record.
Abbreviation: ABOi, ABO-incompatible.

|
We found 32 ABOi transfusion cases in total–nine in the written judgments (cases 1–9), 16 in the KOHEVIS reports (cases 9–24), and nine in literature published in Korea (cases 9 and 25–32) [
7-
12]. Data of the ABOi transfusion cases reported in Korea are presented in
Table 1. Four cases died, 23 cases survived, and the transfusion outcomes of five cases were unknown. All four fatal ABOi transfusion cases were found in written judgments, and case 9 was also found in the 2016 KOHEVIS annual report and the literature [
7]. The types of blood products and errors related with ABOi transfusion are shown in
Fig. 2A and B.
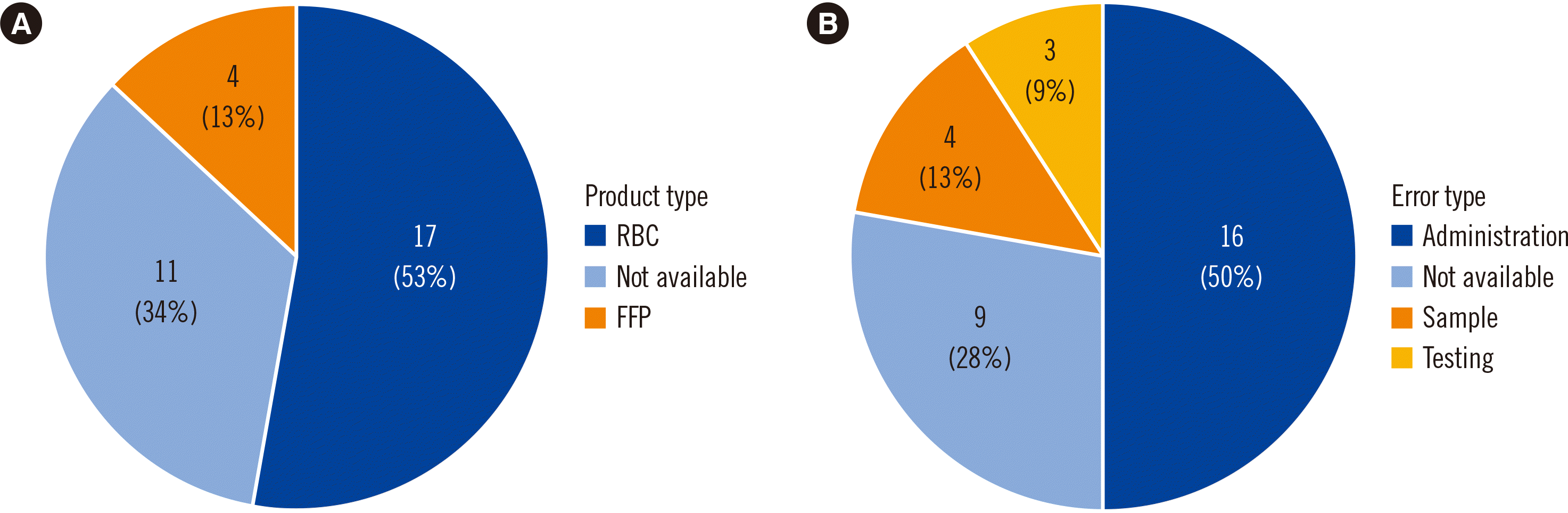 | Fig. 2
Prevalence of ABOi transfusion events according to the (A) type of blood product and (B) type of error.
Abbreviations: ABOi, ABO-incompatible, FFP, fresh frozen plasma; RBC, red blood cells.

|
Table 1
Cases of ABO-incompatible (ABOi) transfusions identified from Korean written judgments (cases 1-9), the Korean hemovigilance system (KOHEVIS; cases 9-24), and the Korean literature (cases 9 and 25-32)
|
No. |
Product type |
ABO |
Transfused volume |
Disease or surgery |
Sex/age (yr) |
Clinical course |
Error |
Reference |
|
Donor |
Recipient |
Sx |
Lab |
AKI |
DIC |
Death |
|
Case 1 |
- |
A |
B |
200 mL |
Ureter stone |
M/27 |
Y |
- |
- |
Y |
Y |
Administration |
Written judgment 1 (1965) |
|
Case 2 |
RBC |
A |
B |
80 mL |
LC |
M/43 |
- |
- |
- |
- |
N |
Administration |
Written judgment 2 (1995) |
|
Case 3 |
FFP |
B |
O |
10 mL |
LC |
M/48 |
N |
- |
- |
- |
N |
Administration |
Written judgment 3 (1995) |
|
Case 4 |
RBC |
B |
A |
200 mL |
Cervix cancer |
F/45 |
- |
- |
- |
- |
N |
Administration |
Written judgment 4 (1995) |
|
Case 5 |
FFP |
O |
A |
2-3 mL |
AML |
M/38 |
- |
- |
- |
- |
N |
Administration |
Written judgment 5 (1995) |
|
Case 6 |
RBC |
B |
O |
40 mL |
Rectal cancer |
M/68 |
Y |
- |
- |
- |
N |
Testing |
Written judgment 6 (1995) |
|
Case 7 |
RBC |
A |
B |
60 mL |
LC |
M/57 |
Y |
- |
- |
- |
Y |
Administration |
Written judgment 7 (1996) |
|
Case 8 |
- |
AB |
O |
- |
Hip fracture |
M/− |
Y |
- |
- |
- |
Y |
Sample |
Written judgment 8 (2012) |
|
Case 9 |
RBC |
A |
B |
270 mL |
Knee replacement |
F/77 |
Y |
Y |
Y |
Y |
Y |
Administration |
Written judgment 9 (2016)
Lim et al. (2018) [7]
KOHEVIS (2016) |
|
Case 10 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Administration |
KOHEVIS (~2009) |
|
Case 11 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Administration |
|
|
Case 12 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Administration |
|
|
Case 13 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Administration |
|
|
Case 14 |
RBC |
B |
A |
- |
- |
- |
- |
- |
- |
- |
- |
Sample |
|
|
Case 15 |
FFP RBC |
A |
AB |
1 U
7 mL |
- |
−/ Preterm |
- |
- |
- |
Y |
N |
Testing |
KOHEVIS (2010) |
|
Case 16 |
RBC |
- |
- |
- |
- |
M/55 |
Y |
Y |
- |
- |
N |
Administration |
KOHEVIS (2012) |
|
Case 17 |
RBC |
- |
- |
- |
- |
M/75 |
Y |
Y |
Y |
Y |
N |
- |
|
|
Case 18 |
RBC |
- |
- |
150 mL |
Infective endocarditis |
M/49 |
N |
Y |
- |
- |
N |
Administration |
KOHEVIS (2013) |
|
Case 19 |
RBC |
- |
- |
30 mL |
Traumatic subarachnoid hemorrhage |
F/78 |
N |
Y |
- |
- |
N |
- |
KOHEVIS (2014) |
|
Case 20 |
RBC |
AB |
A |
2 U |
Hemoperitoneum |
F/50 |
Y |
Y |
- |
- |
N |
Sample |
KOHEVIS (2015) |
|
Case 21 |
RBC |
- |
- |
30 mL |
Colles’ fracture |
F/79 |
Y |
Y |
N |
N |
N |
Administration |
KOHEVIS (2016) |
|
Case 22 |
RBC |
- |
- |
10 mL |
- |
M/78 |
Y |
Y |
- |
- |
N |
Sample |
|
|
Case 23 |
RBC |
- |
- |
30 mL |
Acute kidney failure |
M/75 |
Y |
Y |
- |
- |
N |
Administration |
KOHEVIS (2017) |
|
Case 24 |
RBC |
- |
- |
70 mL |
Traumatic subdural hemorrhage |
F/62 |
Y |
Y |
- |
- |
N |
- |
KOHEVIS (2018) |
|
Case 25 |
- |
A |
O |
400 mL |
Perineorrhaphy |
F/31 |
- |
Y |
Y |
- |
N |
Clerical (unspecified) |
Kim et al. (1978) [8] |
|
Case 26 |
- |
A |
B |
150 mL |
Cervical cancer |
F/50 |
- |
Y |
Y |
- |
N |
|
|
|
Case 27 |
- |
AB |
O |
250 mL |
Cervical cancer |
F/32 |
- |
Y |
Y |
- |
N |
|
|
|
Case 28 |
- |
B |
A |
250 mL |
Atrial septal defect |
F/51 |
- |
Y |
Y |
- |
N |
|
|
|
Case 29 |
- |
A |
B |
400 mL |
Post-delivery bleeding |
F/38 |
- |
Y |
Y |
- |
N |
|
|
|
Case 30 |
RBC |
B |
O |
60 mL |
Total hip replacement |
M/60 |
Y |
Y |
N |
Y |
N |
Administration |
Lim et al. (2004) [9] |
|
Case 31 |
Plasma |
A |
Cis-AB |
2 U |
Intestinal obstruction |
F/70 |
N |
Y |
N |
Y |
N |
Testing |
Woo et al. (2006) [10] |
|
Case 32 |
RBC |
O |
A |
1 U |
Multiple trauma |
M/57 |
- |
Y |
- |
Y |
N |
- |
Kim et al. (2018) [12] |

ABOi transfusion remains one of the main causes of death due to AHTR [
1,
13]. However, data on the frequency of ABOi transfusions are limited. Based on written judgments on ABOi transfusion-related mortality, Cho,
et al. [
14] suggested that there may be unidentified cases of ABOi transfusion in Korea. Kwon,
et al. [
15] suggested that the limited number of ABOi transfusion cases in Korea compared with those in the UK and Japan may be due to underreporting. A concealment culture and a lack of awareness of adverse events among healthcare professionals and related stakeholders have been suggested as major causes of underreporting [
16].
The KOHEVIS is a well-established system, and the numbers of participating hospitals and archived cases have increased from 99 and 458, respectively, in 2009 to 231 and 3,504 in 2019, respectively [
3]. The increased number of reports is probably due to an increase in the awareness of erroneous reporting [
17]. However, we found unreported ABOi transfusion cases after the launch of the KOHEVIS. For example, case 8, which occurred in 2012 and was reported in 2018 by Kim,
et al. [
12], was not identified in the 2012 KOHEVIS annual report. In addition, three ABOi transfusion cases, not described in this report, were confirmed through internet news searches. In the first case, type A and AB blood donor cards were changed, and platelets derived from whole blood were transfused to a man with blood type A (approximately 70 mL) and a man with blood type AB (approximately 40 mL) [
18]. In the second case, type A platelets were transfused to a 54-year-old woman with blood type B. In the third case, type B transfusion products were administered to a 37-year-old woman with blood type O [
19]. Such underreporting of ATRs can be due to a lack of awareness and education among healthcare professionals [
15]. Therefore, it is important to provide regular education and well-designed ATR-reporting forms [
14].
In ABOi transfusion, the most important aspect of management is prevention. Educational programs for all the staff involved in transfusion may be helpful. Cho,
et al. [
14] suggested double checking each event of blood transfusion to prevent ABOi transfusion through the “2-2-2 safe blood transfusion campaign.” This requires two blood samples each for ABO typing and crossmatching, front and back typing by two laboratory personnel, and identification of the patient and blood product by two people prior to transfusion. In addition to the educational programs, electronic systems and barcoding patient identification technology have also been explored to improve patient safety and prevent ABOi transfusion [
20]. If an ABOi transfusion event occurs or is suspected, transfusion should be stopped immediately, and conservative treatment should be performed. AHTRs caused by ABOi transfusion, although alarming and potentially lethal, are self-limited in cases of minor ABOi transfusion (i.e., transfer of FFP/platelets from a type A patient to a type B one).
This study had several limitations. First, we used data available in the e-court system and KOHEVIS. Written judgments dated before 1997 were limited to those of the Supreme Court, and there were no hemovigilance data prior to the establishment of KOHEVIS in 2008. Second, the data were difficult to analyze, because there was insufficient information on the blood type, product type, infusion volume, and outcomes in each case.
In conclusion, we comprehensively analyzed the status of ABOi transfusions in Korea by searching various sources and found eight ABOi transfusion cases in written judgments that were not previously reported to KOHEVIS or in the literature. Our results would contribute to the understanding of the real ABOi transfusion incidence in Korea and the research to promote safe transfusion in the future.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download