This article has been
cited by other articles in ScienceCentral.
Abstract
Congenital factor X (FX) deficiency is a rare autosomal-recessive disease that induces bleeding disorder. Herein, we present the 10-year posttransplant course of a pediatric patient who underwent liver transplantation (LT) with portal vein (PV) stenting for correction of severe congenital FX deficiency, with focus on long-term maintenance of coagulation function and patency of PV stenting. A 17-month-old infant with recurrent hemorrhagic episodes due to FX deficiency underwent split LT using a left lateral section graft. The graft-recipient weight ratio was 2.2%. The graft implantation procedures were performed by following the standard pediatric split LT procedure. Nevertheless, a wall stent was inserted due to PV anastomotic stenosis on posttransplant day 1. Graft function recovered slowly because of partial parenchyma infarct, and the patient was discharged at 46 days after LT operation. The FX activity started to increase soon after LT and gradually normalized; the coagulation profiles have been maintained well for the past 10 years. The patient has been doing well for the past 10 years after LT without any episodes of abnormal bleeding. Due to the risk of vascular complications owed to PV stenting, life-long follow-up is mandatory with special attention until attainment of complete physical growth to adolescent and adulthood.
Go to :

Keywords: Coagulation factor, Autosomal-recessive genetic disease, Portal vein, Stent, Split liver transplantation
|
HIGHLIGHTS |
We present the posttransplant course of a pediatric patient who underwent split liver transplantation with portal vein stenting for correction of severe congenital factor X deficiency. The patient has been doing well for the past 10 years after transplantation without any episode of abnormal bleeding or vascular complication.
|
Go to :

INTRODUCTION
Congenital factor X (FX) deficiency is a rare autosomal recessive genetic disease that induces bleeding disorder. Patients with moderate-to-severe FX deficiencies may have symptoms similar to that of hemophilia A and B, including hemarthrosis, intracranial hemorrhage, and gastrointestinal bleeding [
1,
2]. The diagnosis can be confirmed by an FX assay. Fresh frozen plasma (FFP) and prothrombin complex concentrates have been used as a temporary treatment for bleeding symptoms.
We previously reported our first case of liver transplantation (LT) for correction of severe congenital FX deficiency in a 17-month-old baby with recurrent hemorrhagic episodes [
3]. In our previous case, the patient underwent portal vein (PV) stenting due to anastomotic stenosis one day after LT operation. We herein present the 10-year posttransplant course of the same patient with a focus on long-term maintenance of coagulation function and long-term patency of PV stenting.
Go to :

CASE REPORT
This study was approved by the Institutional Review Board of Asan Medical Center, and the requirement for informed consent was waived.
The patient was born through a full-term normal spontaneous vaginal delivery. At 1 month of age, generalized tonic-clonic seizure developed due to subdural hemorrhage and abscess. She underwent craniectomy for removal of brain abscess, and at that time she was diagnosed with factor X (FX) deficiency. Preoperative laboratory tests revealed severe coagulation prolongation (prothrombin time, 4.2%; international normalized ratio [INR], 13.5; and activated partial thromboplastin time [aPTT], 106.4 seconds). The coagulation factors analysis revealed FX activity as 1%, but activities of other coagulation factors were within the normal range. After treatment with craniectomy, she experienced another episode of subdural hematoma with a seizure. Thereafter, the patient also suffered from recurrent hematochezia and received a frequent transfusion of FFP. Prothrombin complex concentrates are not available at that time in Korea, thus we decided to perform liver transplantation (LT) at the age of 10 months.
The patient’s parents were not able to donate their liver as her mother had deceased FX activity and her father was ABO-incompatible and the size of the left lateral section was too large for this patient. The genetic study of FX showed a novel nonsense mutation, c.[310C<T] (p.[Gln104X]), which was inherited from her mother. The patient was enrolled at the pediatric waiting list for deceased donor LT.
After waiting for 7 months in the waiting list, split LT was allocated at the age of 17 months with a body weight of 11.4 kg. The donor was a 21-year-old 50 kg-weighing male. The left lateral section graft weighed 245 g; thus, the graft-recipient weight ratio was 2.2%. The surgical procedures were performed by following the standard pediatric split LT for left lateral segment graft implantation, which was the same as that of pediatric living donor LT. The recipient PV appeared to be normal (
Fig. 1), thus the recipient PV stump was directly anastomosed to the graft PV. The hepatic artery was reconstructed under surgical microscopy. The duct-to-duct anastomosis was performed for biliary reconstruction. The explant liver showed normal-looking hepatic parenchyma with occasional intracytoplasmic cholestasis (
Fig. 2).
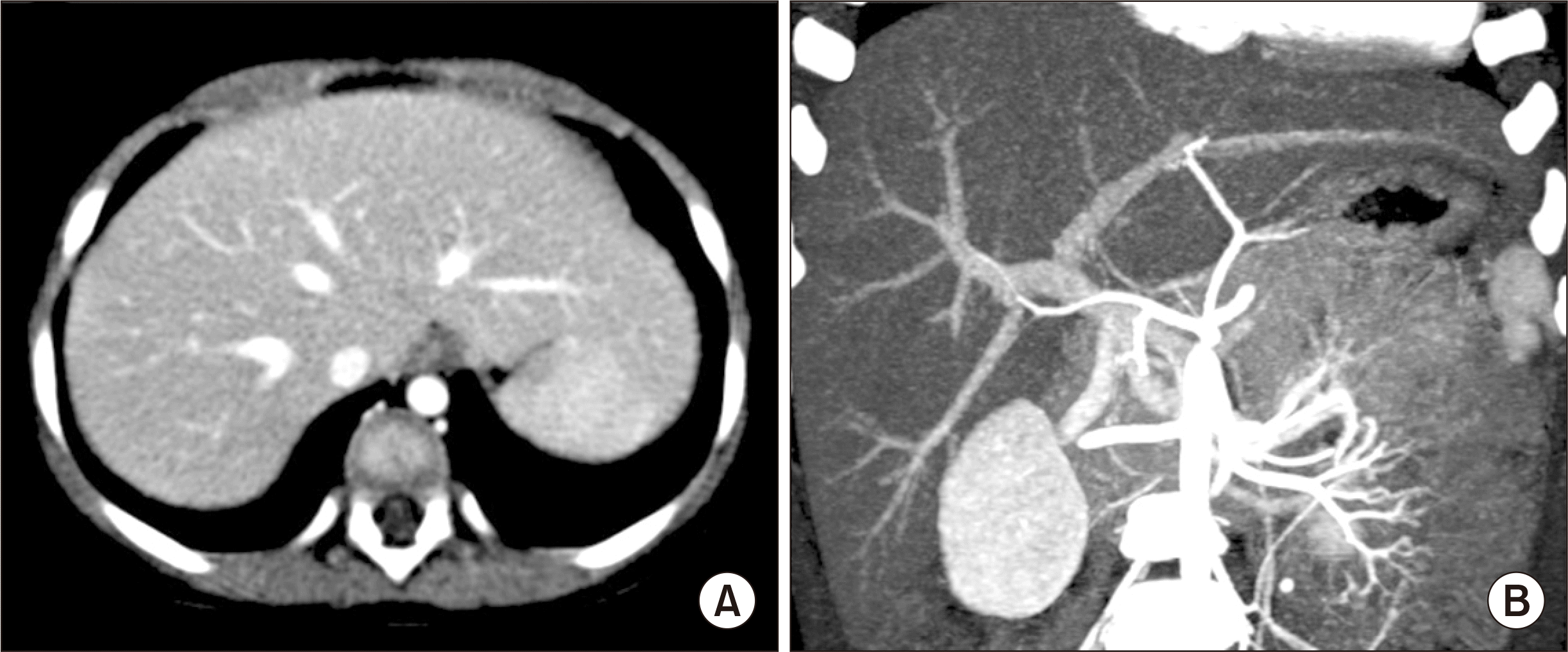 | Fig. 1Pretransplant computed tomography scan showing normal looking hepatic parenchyma (A) and perihilar vascular anatomy (B). 
|
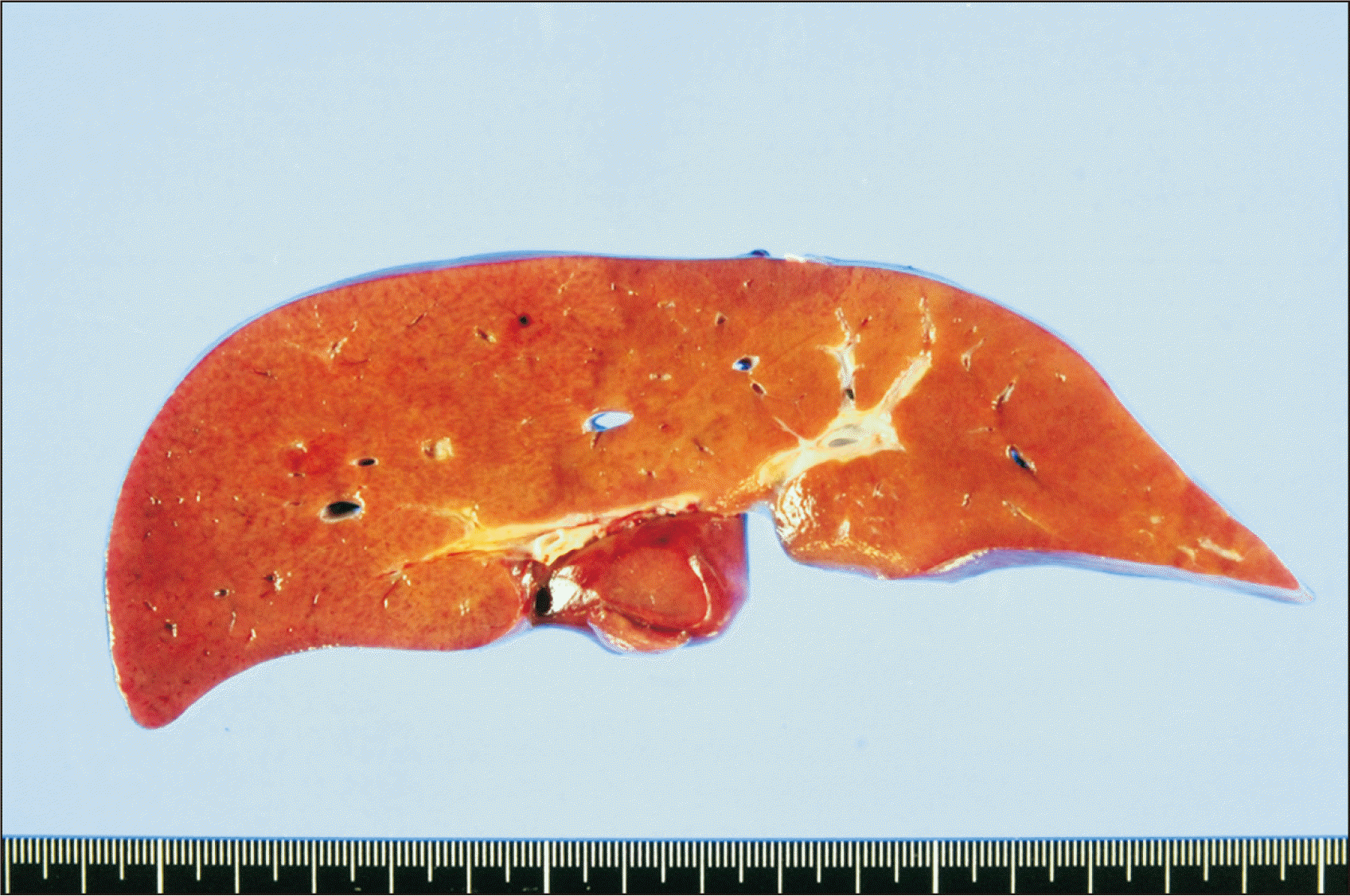 | Fig. 2Gross photographs of the explant liver showing normal-looking hepatic parenchyma. 
|
Doppler ultrasonography performed at posttransplant day 1 showed weak portal flow implicating anastomotic stenosis. Dynamic liver computed tomography revealed segmental stenosis of the PV anastomosis site and heterogeneous low attenuation of the hepatic parenchyma (
Fig. 3). Emergency laparotomy was performed and a 10 mm×40 mm-sized wall stent was inserted across the PV stenosis (
Fig. 4). Follow-up computed tomography and Doppler ultrasonography showed restoration of the PV blood flow (
Fig. 5). Graft function recovered slowly because of partial parenchyma infarct, and the patient was discharged at 46 days after LT operation. The FX activity started to increase soon after LT and gradually normalized at the time of discharge.
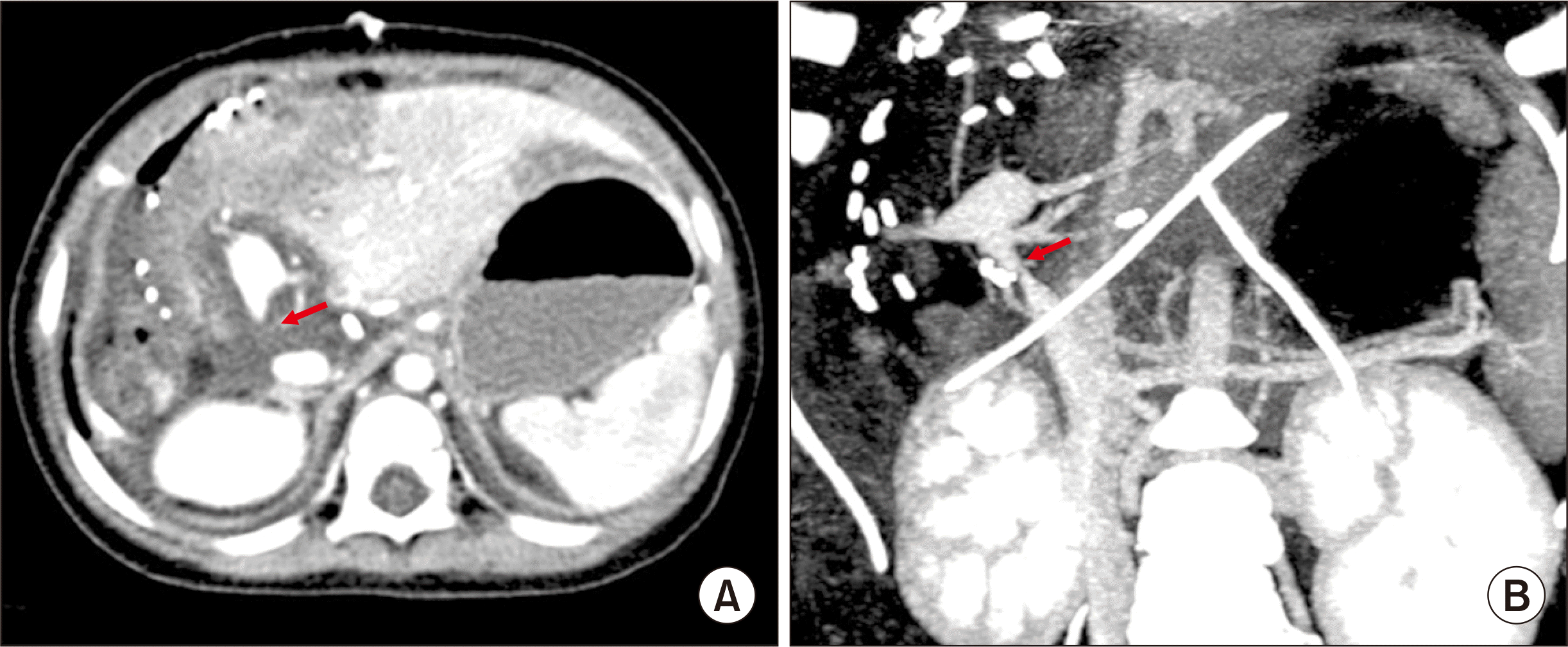 | Fig. 3Posttransplant computed tomography scan taken at 1 day after transplantation. Heterogeneous low attenuation of the hepatic parenchyma (A) and segmental stenosis of the portal vein anastomosis site (B) are identified. Arrows indicate the site of anastomotic stenosis. 
|
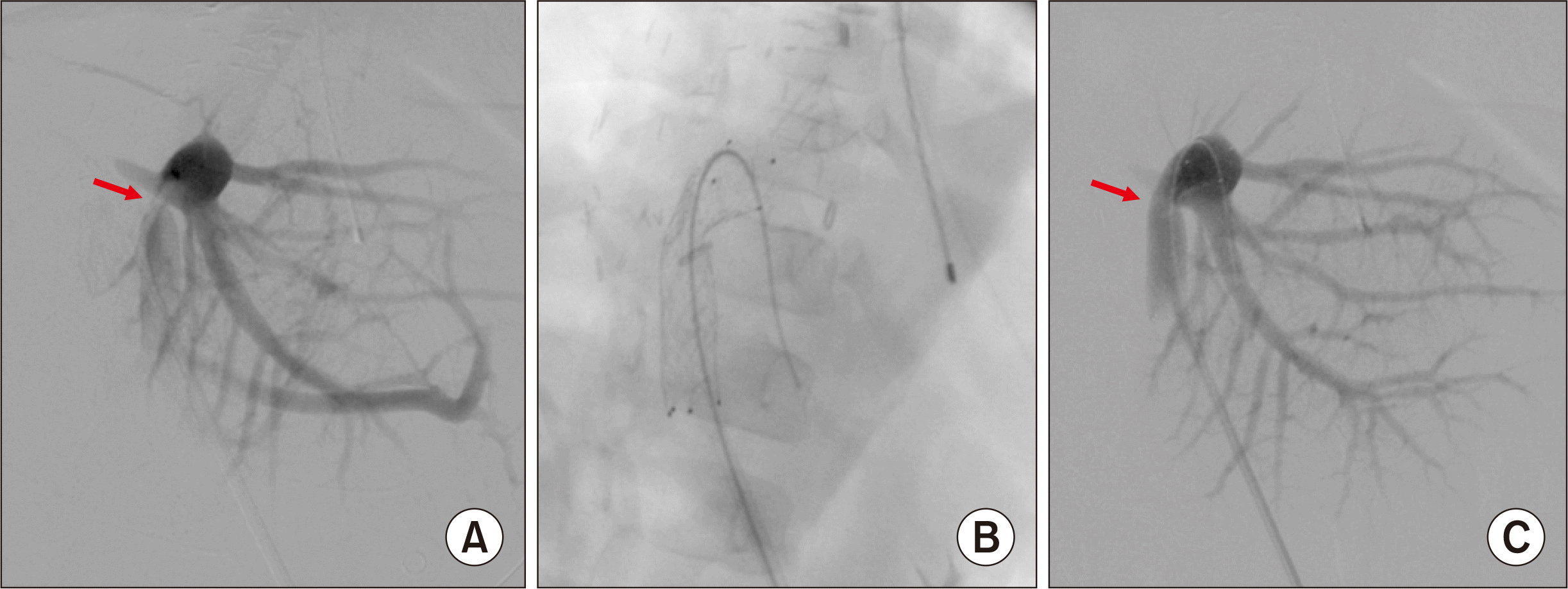 | Fig. 4Posttransplant portal vein stenting performed at 1 day after transplantation. (A) A significant stenosis portal vein anastomosis site is identified. (B) A 10 mm×40 mm-sized wall stent is inserted across the stenosis. (C) The stenotic site is expanded after stenting. Arrows indicate the site of anastomotic stenosis. 
|
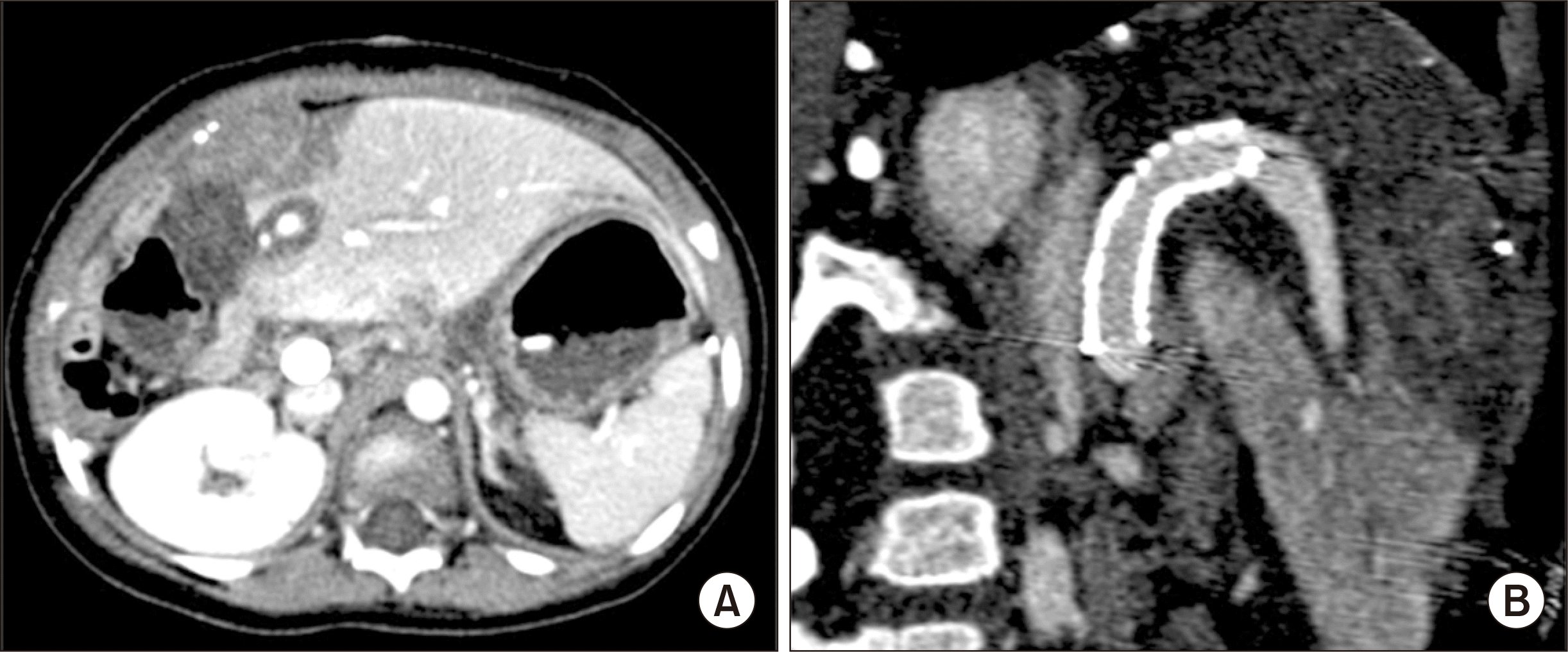 | Fig. 5Posttransplant computed tomography scan taken at 2 days after transplantation. (A) Heterogeneous low attenuation of the hepatic parenchyma was slightly improved. (B) The configuration of the reconstructed portal vein with an internal stent implicates the presence of tension and acute angle bending at the anastomosis site before stenting. 
|
At 3 months after LT, she was readmitted due to Epstein-Barr virus hepatitis, which was uneventfully treated. She has been undergoing Doppler ultrasonography annually to check the patency of the PV stent, and it was observed that PV flow was patent with an intrastent diameter of 6–8 mm (
Fig. 6). She has also received liver elastography annually to check the parenchymal status due to the potential risk of PV insufficiency from significant luminal narrowing of the PV stent, in which the consistency of the liver parenchyma appeared to be not fibrotic. The patient has been doing well for the past 10 years after LT without any episodes of abnormal bleeding. The most recent coagulation test showed prothrombin time 77.2%, INR 1.15, and aPTT 30.8 seconds. We plan to follow up the flow of PV stent at least until full physical growth to adolescent and adulthood is achieved.
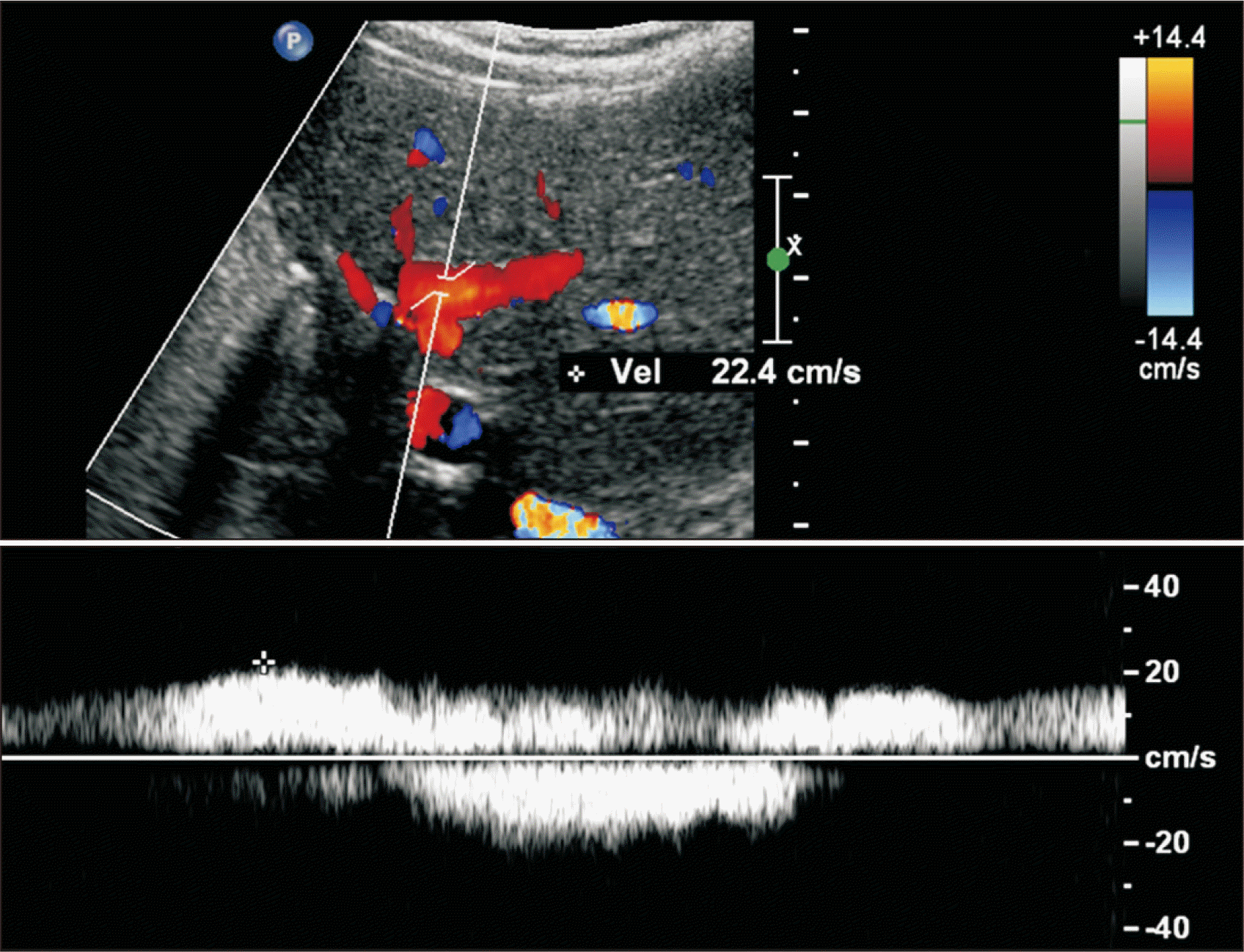 | Fig. 6Follow-up Doppler ultrasonography taken at 9 years after transplantation. The intrahepatic portal blood flow is well maintained (up to 20 cm/sec) with slight narrowing of the intrastent diameter. The intrastent diameter of the portal vein was about 6 mm, which remained unchanged during last 5 years despite gradual physical growth of the patient. 
|
Go to :

DISCUSSION
FX is a vitamin K-dependent, liver-produced serine protease that serves a pivotal role in blood coagulation as the first enzyme in the common pathway to fibrin formation [
1,
2]. FX deficiency is a rare autosomal recessive coagulation disorder [
1]. The estimated prevalence of FX deficiency is about 1/1,500,000 or 2,000,000, therefore the condition is considered as one of the rarest coagulation disorders [
4].
Based on the plasma levels of FX coagulant activity measured through prothrombin time-based assay, patients have been classified into three groups: severe (FX:C, <1%), moderate (FX:C, 1%–5%), and mild (FX:C, 6%–10%). Severe clinical symptoms, such as intracranial hemorrhage, gastrointestinal bleeding, and hemarthrosis are uncommon in patients with FX:C level of >2% [
1]. Intracranial hemorrhage is the most severe complication and an important cause of death in patients with FX deficiency. Intracranial hemorrhage usually occurs in infants between 1 and 5 months of age without any history of trauma [
5]. Our patient had moderate FX deficiency (FX:C level is 1%) and experienced subdural hematoma episodes without trauma as the initial presentation and thereafter frequent development of hematochezia was observed.
FX can be replaced with FFP or prothrombin complex concentrate, but at that time, prothrombin complex concentrate was not available in Korea. Prothrombin complex concentrate is a medication made up of blood clotting factors II, IX, and X. After LT, severe coagulation prolongation and FX activity were completely corrected in our patient. To the best of our knowledge, there exists no other report on LT performed solely for FX deficiency in the literature probably because FX deficiency can be conservatively managed with FFP or prothrombin complex concentrate. There was only one case of a 54-year-old man with FX deficiency and hepatitis C cirrhosis, who underwent LT owing to hepatic encephalopathy [
6]. Currently, congenital FX deficiency is not regarded as an eligible indication of LT.
The LT procedures with a split left lateral section graft was successful in this patient, but early PV stenting was required due to severe stenosis of the PV anastomotic site. In pediatric LT, PV stenosis is one of the most common but most critical complications. Our patient had the normal PV anatomy, unlike PV hypoplasia in pediatric patients with biliary atresia, thus direct PV anastomosis can be regarded as the standard technique. Analysis of 1-day computed tomography and direct portography suggested that the underlying causes of PV stenosis were sharp narrowing at the anastomotic site per se and anastomotic site tension at the reconstructed PV, which were not recognized during LT operation probably due to lack of the surgeons’ experience on pediatric LT. There exists no incidence of the occurrence of this type of PV stenosis for a long time in our pediatric LT program after our adoption of funneling venoplasty and interposition of vein conduit homograft for PV reconstruction [
7-
9]. If there is a great discrepancy in sizes of the graft and recipient PVs or anastomotic site tension is anticipated, the interposition of a vein conduit homograft is indicated [
8,
9].
PV stenting is the last therapeutic procedure to save the life of infant patients, similarly to our patient, thus it should be avoided as much as possible. The PV stent does not expand along with the growth of patients, from infant to adolescent and adulthood [
10-
12]. In our patient, PV blood flow through the PV stent might be sufficient for a few years after LT, but we are concerned that it can be insufficient with the patient’s growth. PV stenting during the infant period is one of the main causes of late retransplantation. Thus, we have to pay special attention to the patency of PV stent and PV blood flow to the liver through regular surveillance with Doppler ultrasonography and liver elastography.
In conclusion, we present the first 10 years posttransplant course of LT in a pediatric patient with severe congenital FX deficiency. Although coagulation profiles are corrected completely, the vascular complication with PV stenting remains. Thus life-long follow-up surveillance is mandatory with special attention, at least until full physical growth to adolescent and adulthood is achieved.
Go to :

ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2021-00-03002-010).
Author Contributions
Conceptualization: SH. Data curation: JMN, KMK, SHO. Methodology: DYK, TYH, GWS, DHJ. Visualization: SH. Writing–original draft: SH, JMN. Writing–review & editing: SH.
Go to :

REFERENCES
3. Bang SH, Oh SH, Kim KM, Song SM, Seo JJ, Yoo HW, et al. 2012; Successful liver transplantation for a child with life-threatening recurrent bleeding episodes due to congenital factor X deficiency: a case report. Transplant Proc. 44:583–4. DOI:
10.1016/j.transproceed.2012.01.075. PMID:
22506295.


4. Girolami A, Scandellari R, Scapin M, Vettore S. 2008; Congenital bleeding disorders of the vitamin K-dependent clotting factors. Vitam Horm. 78:281–374. DOI:
10.1016/S0083-6729(07)00014-3. PMID:
18374200.


5. Senturk S, Guzel E, Bayrak AH, Bukte Y, Guzel A. 2010; Factor X deficiency presenting with bilateral chronic subdural hematoma. Pediatr Neurosurg. 46:54–7. DOI:
10.1159/000315004. PMID:
20516741.


6. Gordon FH, Mistry PK, Sabin CA, Lee CA. 1998; Outcome of orthotopic liver transplantation in patients with haemophilia. Gut. 42:744–9. DOI:
10.1136/gut.42.5.744. PMID:
9659174. PMCID:
PMC1727096.



7. Kang SH, Hwang S, Jung DH, Ahn CS, Moon DB, Ha TY, et al. 2013; Unification venoplasty to cope with recipient portal vein anomaly during living donor liver transplantation. Transplant Proc. 45:3000–4. DOI:
10.1016/j.transproceed.2013.08.073. PMID:
24157023.


8. Hwang S, Kim DY, Ahn CS, Moon DB, Kim KM, Park GC, et al. 2013; Computational simulation-based vessel interposition reconstruction technique for portal vein hypoplasia in pediatric liver transplantation. Transplant Proc. 45:255–8. DOI:
10.1016/j.transproceed.2012.05.090. PMID:
23375311.


9. Namgoong JM, Hwang S, Oh SH, Kim KM, Park GC, Ahn CS, et al. 2020; Living-donor liver transplantation with inferior vena cava replacement in an infant recipient with advanced hepatoblastoma. Ann Hepatobiliary Pancreat Surg. 24:72–7. DOI:
10.14701/ahbps.2020.24.1.72. PMID:
32181433. PMCID:
PMC7061035.



10. Cho YP, Kim KM, Ha TY, Ko GY, Hwang JY, Park H, et al. 2014; Management of late-onset portal vein complications in pediatric living-donor liver transplantation. Pediatr Transplant. 18:64–71. DOI:
10.1111/petr.12204. PMID:
24341631.


11. Shim DJ, Ko GY, Sung KB, Gwon DI, Ko HK. 2018; Long-term outcome of portal vein stent placement in pediatric liver transplant recipients: a comparison with balloon angioplasty. J Vasc Interv Radiol. 29:800–8. DOI:
10.1016/j.jvir.2017.11.019. PMID:
29545104.


12. Yeh YT, Chen CY, Tseng HS, Wang HK, Tsai HL, Lin NC, et al. 2017; Enlarging vascular stents after pediatric liver transplantation. J Pediatr Surg. 52:1934–9. DOI:
10.1016/j.jpedsurg.2017.08.060. PMID:
28927979.


Go to :









 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download