This case was approved by the Institutional Review Board of the Yeungnam University Hospital (IRB No. 2020-06-002). The board waived the need for informed consent since the subjects’ records and information were anonymized and de-identified prior to the analysis.
A 60-year-old man with a history of diabetic nephropathy, hypertension, and heart failure was hospitalized following an ABO-incompatible kidney transplant from a living unrelated donor at our hospital in August 2019. A month before the transplant, rituximab injection was administered. ABO antibody titers tested 2 weeks before transplantation were 1:64 for anti-A and 1:8 for anti-B. The recipient’s blood type was Rh (+) O, and the donor’s blood type was Rh (+) A. Plasma exchange was performed every other day until the antibody titers were reduced to 1:8. One day before surgery, anti-A titer of 1:16 and an anti-B titer of 1:8 were reached; the surgery proceeded as scheduled. His blood urea nitrogen (BUN) level was 62 mg/dL and serum creatinine level was 8.47 mg/dL. Immunosuppressants, including methylprednisolone, mycophenolate mofetil, and tacrolimus, were administered 1 week before surgery. Immediately after surgery, his serum creatinine was 7.09 mg/dL and urine output was maintained above 100 mL/hr. On postoperative day (POD) 1, his BUN was 35 mg/dL and serum creatinine was 3.08 mg/dL. On POD 3, his urine output suddenly decreased to 30 mL/hr and serum creatinine level increased to 3.84 mg/dL. The trough level of tacrolimus was 9.37 μg/mL. Generalized edema gradually worsened and an increased dose of intravenous furosemide up to 60 mg was administered to maintain a urine output of 100 mL/hr. On POD 4, his serum creatinine was 4.3 mg/dL. With a normal duplex kidney scan, allograft rejection could not be ruled out. A biopsy of the transplanted kidney was therefore indicated, and steroid pulse therapy (methylprednisolone 250 mg q 12 hours) was started in advance. However, 3 days of steroid treatment were ineffective, and his serum creatinine levels increased to 4.6 mg/dL (
Fig. 1). There were no abnormalities in donor-specific antibody (DSA) and antibody titers—ABO titers were 1:1 for anti-A and 1:4 for anti-B, respectively; C3/C4 levels were in the normal range, while antineutrophil cytoplasmic antibodies and fluorescent antinuclear antibody tests were negative. Eventually, the urine output decreased to zero. This was accompanied by worsening pulmonary edema and generalized edema. Although we were unable to confirm the biopsy results, plasma exchange and administration of intravenous immunoglobulin (IVIG) 100 mg/kg were started and performed every other day. After three rounds of plasma exchange, his serum creatinine began to decrease gradually to 2.3 mg/dL. However, it did not improve further and increased again to 2.6 mg/dL. The renal biopsy showed no signs associated with tissue injury in the glomerulus and tubulointerstitium of the cortex. C4d deposits were observed in both the cortex and medulla (
Fig. 2). However, acute tubular injury, neutrophilic peritubular capillaritis (Banff PTC grade 3), and interstitial hemorrhage were observed only in the medulla (
Fig. 3). The kidney biopsy findings were compatible with AMR, except that the lesion was limited to the medulla. We decided to start bortezomib therapy (1.3 mg/m
2) on days 1, 4, 8, and 11. Four days into treatment, his serum creatinine levels decreased to 1.73 mg/dL. His white blood cell (WBC) count was 9.17 K/μL and platelet count was 89 K/μL. Prior to the second injection, his WBC count was 3.96 K/μL and platelet count was 62 K/μL. The second and third dosages of bortezomib were therefore reduced (1 mg/m
2) owing to thrombocytopenia. Treatment was stopped at the fourth injection owing to neutropenia (WBC, 1.76 K/μL; platelet count, 80 K/μL). Serum creatinine decreased to 1.22 mg/dL. Tacrolimus and prednisolone were maintained as immunosuppressive therapy. Mycophenolate was maintained for 4 days after surgery and then stopped because of thrombocytopenia. The patient was discharged 2 months later with a serum creatinine level of 0.96 mg/dL, WBC count of 5.16 K/μL, and platelet count of 96 K/μL. Six months after surgery, these parameters were maintained to 1.13 mg/dL, 5.76 K/μL, and 168 K/μL, respectively, eventually reaching stability at 9 months after kidney transplantation.
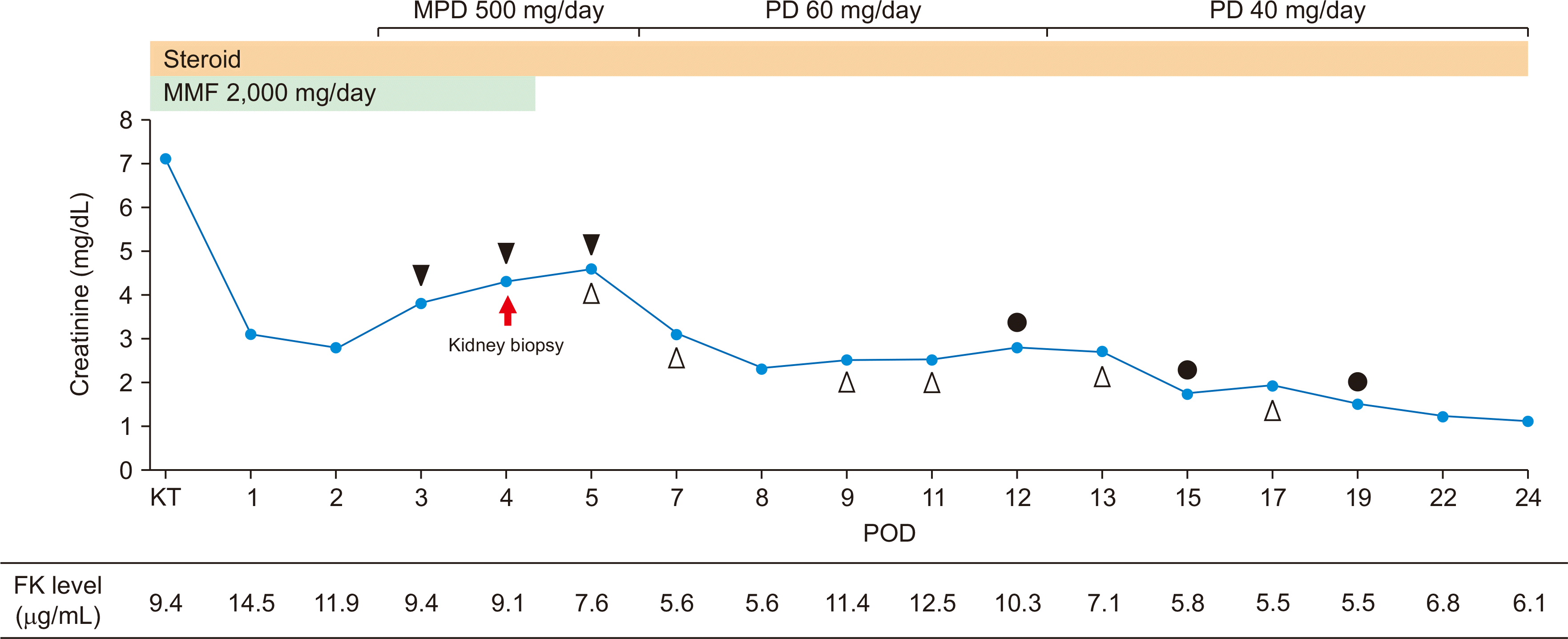 | Fig. 1Changes in creatinine levels after kidney transplantation. A biopsy of the transplanted kidney on postoperative day (POD) 4. Rejection therapy was used, which included steroid pulse therapy (POD 3–5), plasmapheresis (POD 5, 7, 9, 11, 13, and 17), and bortezomib treatment (POD 12, 15, and 19). ▲, steroid pulse therapy; △, plasmapheresis; ●, bortezomib. MPD, methylprednisolone; PD, prednisolone; MMF, mycophenolate mofetil; KT, kidney transplantation; FK, FK-506. 
|
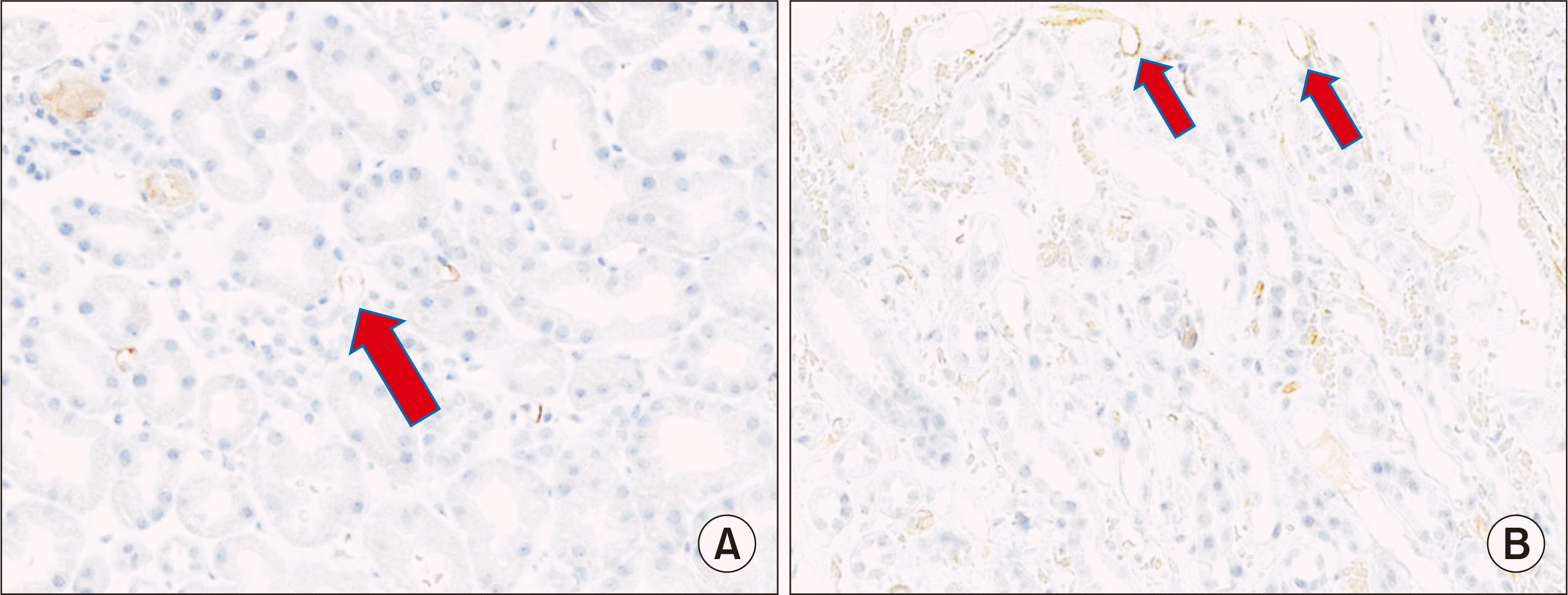 | Fig. 2C4d-positive staining in the cortex (A) and medulla (B). Arrows show immunostaining for C4d (×400). 
|
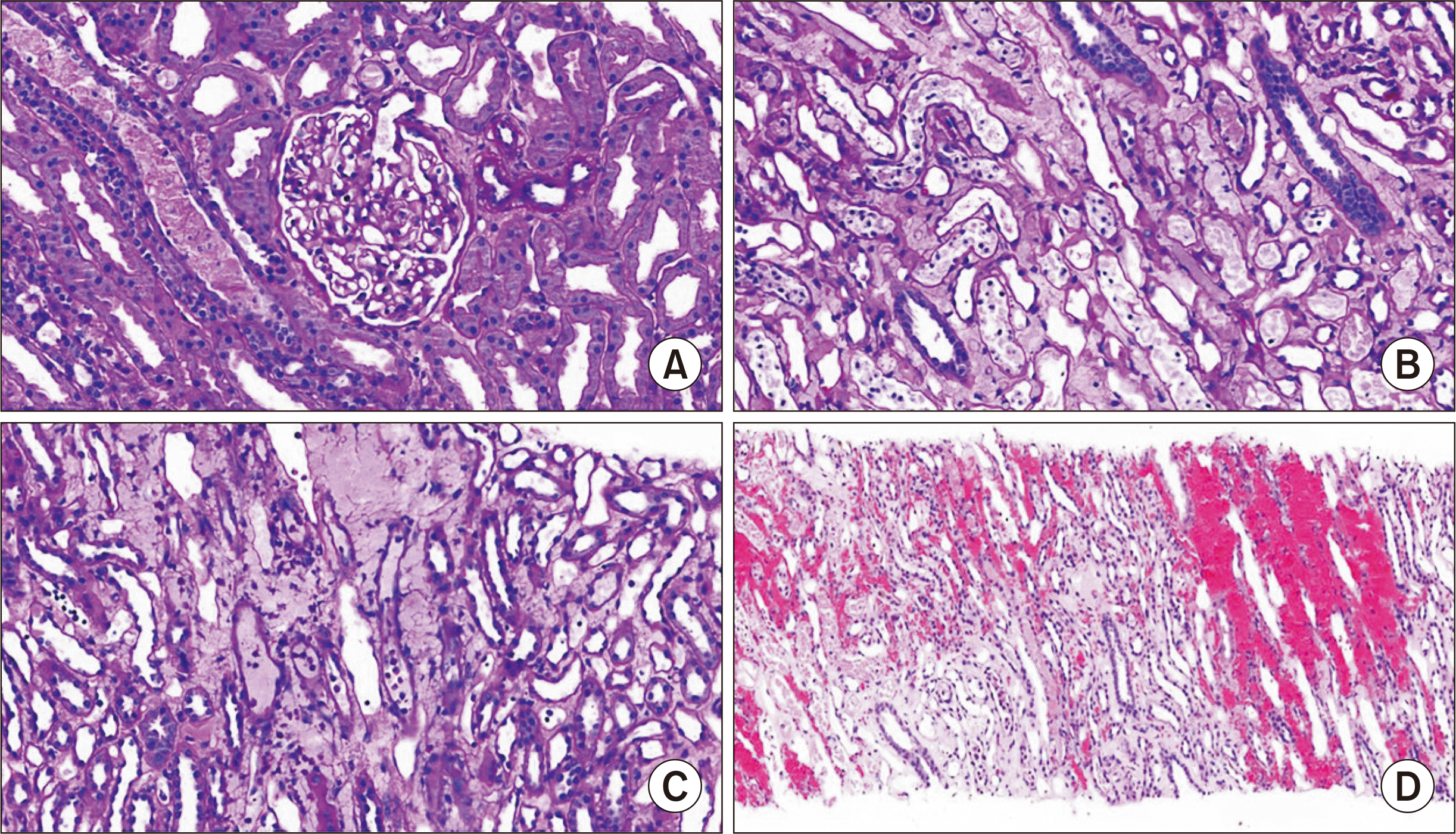 | Fig. 3Light microscopy. (A) No specific findings in the cortex (periodic acid-schiff [PAS], ×400). (B) Acute tubular injury in the medulla (PAS, ×400). (C) Neutrophilic peritubular capillaritis in medulla compatible to PTC grade 3 (PAS, ×400). (D) Interstitial hemorrhage in the medulla (H&E, ×200). 
|





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download