Abstract
Background
The Model for End-Stage Liver Disease (MELD)-based allocation system was implemented in Korea in July 2016 without a mandatory abstinence period for liver transplantation (LT) listing. However, the impact of the allocation policy has not been evaluated in patients with severe alcoholic hepatitis (AH).
Methods
A total of 81 consecutive patients with severe AH between January 2014 and December 2018 were analyzed. The clinical course of patients before and after the implementation of the MELD-based allocation system was assessed.
Results
More patients received LT (25%–65%) after the MELD allocation system was implemented. The increase in patients receiving deceased donor LT was dramatic (17%–51%, P=0.001) compared to patients receiving living donor LT (7%–14%, P=0.30). The overall survival was better for those who received LT (88% vs. 44% at 1 year, P<0.001), and after the MELD era (1-year survival rate 80% vs. 50%, P=0.005). Post-LT mortality was observed in six patients, with one case of mortality related to recidivism. Baseline MELD and steroid response were factors associated with transplant-free survival.
Conclusions
After implementation of the MELD-based allocation system, deceased donor LT dramatically increased in patients with severe AH. This translated into increased overall survival, but at a cost of mortality due to recidivism. Urgent evaluation is warranted to identify criteria to justify the use of precious liver grafts from deceased donors for severe AH patients in Korea.
Alcoholic liver disease represents a spectrum of injuries, ranging from simple steatosis and alcoholic hepatitis (AH) to cirrhosis [1]. AH is a clinical syndrome that affects patients with chronic and active harmful alcohol consumption and is associated with high mortality [2,3]. The modified discriminant function (MDF) score has been widely used to stratify AH severity [1]. Severe AH is defined as an MDF score ≥32 and is associated with a survival rate of 50%–65%, while patients with an MDF score <32 is associated with a survival rate of 90% [4].
To date, the management of severe AH remains a clinical challenge with few effective treatment options [5]. The cornerstone therapy for AH is nutrition and abstinence from alcohol [3]; however, this is not often successful. Corticosteroids are the only treatment option shown to reduce mortality from severe AH [6]. However, the benefits of corticosteroid treatment remain controversial [7]. Corticosteroids administered to patients with severe AH increase the risk of developing serious infections and may increase the risk of mortality in patients who experience infection [8]. Patients with severe AH unresponsive to medical therapy have 1-year mortality rates of up to 70%–90% [9,10]. Early liver transplantation (LT) for severe AH is a potentially life-saving treatment with established survival benefits [11,12]. However, LT for AH as a rescue therapy is also controversial [13,14], especially in terms of the selection criteria justifying the use of precious liver grafts [15,16].
In Korea, the liver allocation system for deceased donor LT (DDLT) has been based on the Child-Turcotte-Pugh score; however, to increase objectivity and equality of allocation [17], a Model for End-Stage Liver Disease (MELD)-based allocation system was implemented in June 2016 [18]. The change in the liver allocation system has had a huge impact on potential LT candidates, including patients with severe AH. However, the impact of the allocation system change on the outcomes of patients with severe AH has not been assessed. Therefore, in this study, we compared the clinical outcomes of patients with severe AH before and after the MELD-based allocation system to determine the impact of the change in allocation system on the outcomes of patients with severe AH in Korea.
The study protocol was approved by the Ethics Committee of the Samsung Medical Center (IRB No. 2019-07-118). Since this study used only de-identified data routinely collected during hospital visits, the requirement to obtain informed patient consent was waived.
This study was a single-center, retrospective cohort study conducted at the Samsung Medical Center, Seoul, South Korea. We screened 886 patients who were admitted to our liver unit without malignancy, viral hepatitis, autoimmune hepatitis, or primary biliary cirrhosis between January 2014 and December 2018. Among them, we identified 81 severe AH patients who met the diagnostic criteria of definite or probable AH by the National Institute on Alcohol Abuse and Alcoholism [19]. In brief, definite AH is defined for patients meeting the clinical diagnosis criteria of AH with biopsy confirmation. Probable AH is defined for patients meeting the clinical diagnosis criteria of AH in the absence of potential confounding factors without biopsy confirmation. The criteria for the clinical diagnosis of AH are as follows: (1) onset of jaundice (serum total bilirubin >3.0 mg/dL) within the previous 8 weeks; (2) ongoing consumption of >40 g (female) or >60 g (males) of alcohol/day for 6 months or more with less than 60 days of abstinence before the onset of jaundice; (3) aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <400 IU/L, AST >50 IU/L, AST/ALT ratio >1.5; (4) absence of other chronic liver diseases (hepatitis B, hepatitis C, autoimmune, or metabolic liver disease); and (5) absence of sepsis, shock, cocaine use, or recent use (within 30 days) of a drug with the potential for drug-induced liver injury. An MDF score ≥32 was used to define severe AH. The pre-MELD era was from January 2014 to June 2016 and the post-MELD era was from July, 2016 to December, 2018 in this study. In our institution, a physician or surgeon determines the necessity of LT for each patient, and when it is deemed necessary, a multidisciplinary team, which includes transplant surgeons, hepatologists, psychologists, cardiologists, infectious disease specialists, and social workers evaluates each patient. After the multidisciplinary evaluation, the respective physician or surgeon has a discussion with the patient and family members regarding registering on the LT waitlist. The transplant coordinator then lists the patient and registers them to the Korean Network for Organ Sharing. Living donor LT (LDLT) is explained in all situations, and evaluations are performed according to the living liver donor selection criteria if there is a willing donor within the family. In our institution, the selection criteria for living liver donors are as follows: (1) <65 years of age; (2) expected remnant liver volume greater than 30%; and (3) no evidence of chronic liver disease. There are no specific criteria for emergent LDLT in the setting of severe AH. When there is a willing liver donor who fulfills the donor selection criteria, transplant surgeons and hepatologists discuss with the patient and family members whether to proceed with emergent LDLT, wait for DDLT allocation, or wait for spontaneous recovery on a case-by-case basis. If there is no willing live liver donor or no liver donor who fulfills the donor selection criteria, the patient waits for DDLT allocation or spontaneous recovery. When a deceased donor is allocated to a patient, transplant surgeons and hepatologists discuss with the patient and family members whether to proceed with emergent DDLT, wait for spontaneous recovery, or decline the allocation due to high risk of transplant futility.
The primary outcome was overall survival before and after the MELD-based allocation system was implemented. The duration of survival was calculated from the date of initial hospitalization to death or the last date of follow-up. The secondary outcome was transplant-free survival (TFS). For TFS, the duration of survival was calculated from the date of initial hospitalization to death, last date of follow-up, or date of LT, whichever came first. The reference day was May 5, 2019.
The following variables were collected by reviewing the electronic medical records of each patient: age at hospitalization; sex; previous decompensation history; presence of infection; gastrointestinal bleeding; imaging, endoscopic, and laboratory results; medication use; and LT during follow-up. The presence of hepatomegaly, splenomegaly, and ascites were evaluated using computed tomography (CT) or ultrasonography findings. Splenomegaly was defined as the largest dimension >11 cm, and hepatomegaly was defined as a longitudinal axis >16 cm at the midclavicular line on CT or ultrasonography. Infections were defined as follows [20]: (1) spontaneous bacteremia: blood cultures without a source of infection; (2) spontaneous bacterial peritonitis: ascites fluid polymorphonuclear cell count >250/uL; (3) lower respiratory tract infection: new pulmonary infiltration in the presence of (i) at least one respiratory symptom (cough, sputum production, dyspnea, pleuritic pain) with (ii) at least one finding on auscultation (rales or crepitation) or one sign of infection (core body temperature >38°C or <36°C, shivering or leucocyte count >10,000/mm3 or <4,000/mm3) in the absence of antibiotics; (4) skin infection: fever with cellulitis; (5) urinary tract infection: urine white blood cell >15/high-power field with either positive urine Gram stain or culture in symptomatic patients; and (6) other source of infection (e.g., intra-abdominal infection, secondary peritonitis). Gastrointestinal bleeding was defined as evidence of hematemesis/melena/hematochezia, a drop in hemoglobin of 2 g/dL or more from baseline, or the need for a transfusion. Previous decompensation included jaundice, ascites, variceal hemorrhage, or hepatic encephalopathy prior to admission. The presence of varix was identified by reviewing endoscopic findings among patients who underwent upper endoscopy and by reviewing CT findings. The following laboratory variables at initial hospitalization were collected: white blood cell count, platelet count, prothrombin time, bilirubin, ALT, AST, gamma glutamyltransferase, blood urea nitrogen, and creatinine. All patients were assessed using the MELD score (3.78 ln [bilirubin]+11.20 ln [international normalized ratio]+9.57 ln [creatinine]+6.43) [21]. For those who received LT, the MELD score on the day of transplantation, waiting time, and alcohol relapse after LT were collected. For those who received corticosteroid treatments, the Lille score (3.19–0.101×age+0.147×albumin on day 0+0.0165×evolution in bilirubin level–0.206×renal insufficiency–0.0065×bilirubin on day 0–0.0096×prothrombin time was calculated. Steroid non-responders were defined as a Lille score >0.45 [22].
Baseline characteristics were described using frequency (percentage) and median (interquartile range). Continuous variables were analyzed using Student t-test or the Mann-Whitney U-test. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. For the MELD score, patients were divided into two groups according to MELD score (<25 and ≥25). The cutoff point was chosen based on the area under the receiver operating characteristic analysis. The survival curves were plotted using the Kaplan-Meier method, and the difference between the curves was tested using the log-rank test. Multivariate Cox regression analysis was performed with all variables with a P-value <0.10 in univariate analysis to evaluate predictors of overall survival and TFS. Statistical significance was set at P<0.05.
The baseline characteristics of the patients are summarized in Table 1. There were no differences in baseline characteristics before and after the MELD-based allocation system was implemented. The proportion of patients receiving LT increased from 25% (10/40) in the pre-MELD era to 65% (27/41) in the post-MELD era (P<0.001). The proportion of patients receiving DDLT increased from 17% (7/40) to 51% (21/41) (P=0.001) in the pre- and post-MELD era, respectively. Although not statistically significant (P=0.30), the proportion of patients receiving LDLT increased from 7% (3/40) to 14% (6/41) in the pre- and post-MELD eras, respectively. When stratified according to MELD score, the DDLT rate did not change significantly (18% to 25%, pre- and post-MELD eras, P=0.53) for those with low MELD scores (<25), while the DDLT rate increased from 17% (pre-MELD era) to 68% (post-MELD era, P<0.001) for those with high MELD scores (≥25) (Fig. 1).
During the follow-up period of a median of 8.4 months (range, 0.1–64.0 months), mortality was observed in 30 patients (37%). Of the 81 patients, 22 (27%) used corticosteroids, while 59 (73%) did not. The proportion of patients who recovered without LT, received LT, and died without LT were 36%, 36%, and 27%, respectively, among the 22 patients who used steroids, and were 20%, 49%, and 30%, respectively, among the 59 patients who did not use steroids (Fig. 2). Overall survival improved in patients with severe AH managed after the MELD-based allocation system compared to before (80% vs. 50% at 12 months, P=0.005) (Fig. 3). In the univariate analysis, LT and the MELD-based allocation system era were factors associated with overall survival (Table 2). Age (per year), male sex, and hepatomegaly showed a marginal association with overall survival. In the multivariate analysis, age (hazard ratio [HR], 1.04; 95% confidence interval [CI], 1.00–1.08; P=0.028) and LT (HR, 0.16; 95%, CI, 0.06–0.52; P<0.001) were independent factors affecting overall survival (Table 2). Patients who received LT showed better overall survival than patients who did not receive LT (88% vs. 44% at 1 year, P<0.001) (Supplementary Fig. 1).
The TFS rates were 42.3% and 32.7% at 3 and 6 months, respectively. In the multivariable analysis, response to corticosteroids (compared to non-users; HR, 0.25; 95% CI, 0.09–0.71; P=0.009), corticosteroid non-response (compared to non-users; HR, 2.07; 95% CI, 1.00–4.30; P=0.050), and MELD score ≥25 at initial hospitalization (compared to <25; HR, 2.79; 95% CI, 1.60–4.88; P<0.001) were independently associated with TFS (Table 3). When stratified according to MELD score and steroid response, the 3-month TFS was 70% and 24% for MELD scores <25 and ≥25, respectively (P<0.001) (Supplementary Fig. 2A), and 38%, 88%, and 16% for steroid non-users, steroid responders, and steroid non-responders, respectively (P=0.005) (Supplementary Fig. 2B).
Among the 37 patients who received LT, 9 received LDLT, and 28 received DDLT. The median time from LT listing was not significantly different between the two groups (LDLT, 14 days vs. DDLT, 9 days; P=0.24). The DDLT group had higher MELD scores at transplantation than the LDLT group (40 vs. 29, P=0.035). After LT, alcohol relapse was identified in 5 of the 37 patients (13%). Six deaths occurred after LT. The cause of mortality was sepsis in four patients, graft rejection in one patient, and liver failure related to recidivism in one patient (Supplementary Table 1).
In this study, we observed improved survival in patients with severe AH in the post-MELD era. There was no significant difference in baseline patient characteristics before and after the MELD allocation system was implemented. However, more patients received LT, and the number of patients who received DDLT increased dramatically (17% to 51%) in the post-MELD era. LT was an independent predictor of survival, while the era (before or after the MELD allocation system) was not an independent factor for survival. All these findings suggest that changing the liver allocation system was the main driver of the dramatic improvement in outcomes for patients with severe AH by increasing the allocation of deceased donors to severe AH patients in Korea.
By definition, patients with severe AH are those with less than 60 days of abstinence before the onset of jaundice [19]. Considering the paucity of donor organs, the abstinence criterion of 6 months has been widely used in transplant centers in Western countries before patients with alcohol-related liver disease will be considered for LT [23,24]. However, most deaths from severe AH occur within 2 months, and early LT without a mandatory 6-month abstinence period can be a lifesaving treatment [16]. The survival benefits of emergent LT for patients with severe AH has been established; however, the post-LT alcohol relapse rate is approximately 25% [4,25,26]. Due to the different social and cultural situation in Korea compared to Western countries, most transplantation centers do not require a certain duration of sobriety before listing an alcohol-related liver disease patient for either LDLT or DDLT. In a recent nationwide study in Korea, there was an increase in the DDLT rate and the proportion of candidates with alcoholic liver disease on the waiting list after the implementation of MELD [27]. In addition, among DDLT recipients, the proportion of patients with hepatocellular carcinoma decreased significantly after the implementation of MELD (from 26.2% to 12.3%, P<0.001) [27]. After the implementation of MELD, the DDLT rate increased from 17% to 68% among patients with MELD ≥25 in this study, while no significant increase in the DDLT rate was observed in patients with low MELD scores (<25). Therefore, the implementation of MELD can improve the outcomes of patients with severe AH by increasing the DDLT rate for patients with high MELD scores; however, this may be at the cost of decreased survival of waitlist patients due to other etiologies. Currently, recidivism after DDLT does occur, and there are no criteria to justify the use of precious liver grafts for patients with severe AH. Hence, studies are urgently needed to identify the potential harms and benefits of MELD implementation from a nationwide perspective in Korea.
In this study, LT was an independent factor associated with survival, and the difference in the survival rate at 1 year was significant (88% vs. 44% at 1 year, P<0.001). This indicates that LT is a lifesaving treatment for patients with severe AH. However, limited organ supply, risk of recidivism, and the fact that a DDLT is a social resource is a major concern for transplanting patients with severe AH [28]. In this study, we observed one case of early death due to recidivism. There is an unmet clinical need for patients with severe AH to justify the use of precious liver grafts in the selection criteria [16]. In this regard, factors associated with TFS can be useful in guiding management plans. In this study, baseline MELD score and corticosteroid response were independent factors associated with TFS. The MELD score has been shown to have high prognostic power in classifying the prognosis of patients with severe AH, albeit with different cutoff values [1]. Those with a high MELD score require careful monitoring for poor prognosis. Another factor was the corticosteroid response, defined by the Lille score. Our findings were consistent with a recent modeling study that showed that early LT can increase life expectancy and can provide the highest benefit when MELD and Lille scores are used in clinical scenarios [29]. Considering that corticosteroid response is represented by the Lille score, the MELD score and corticosteroid response may be used to determine who may benefit from early LT.
Our study has some limitations. First, for inclusion in this study, we used the diagnostic criteria for definite or probable AH by the National Institute on Alcohol Abuse and Alcoholism [19]. These criteria allow patients with previous decompensation to be diagnosed with AH. However, it can be very challenging to deferentiate severe AH from acute decompensation of decompensated cirrhosis, and misclassification can occur if a liver biopsy is not performed [19]. Of note, previous decompensation was observed in 46%, while liver biopsy was performed in only 23% of the patients analyzed in this study. Hence, misclassification bias may exist. Second, data were obtained from a retrospective study conducted at a single institution with potential selection bias. The decisions regarding steroid treatment and LT were made by the doctor in charge of the patient; therefore, unmeasured or unidentified bias might be present in the decision to provide steroid treatment or list the patient for LT. Additionally, assessment of alcohol consumption after LT was retrospectively collected from medical records, so there is a possibility of underestimation of alcohol use due to patient underreporting. The sample size of this study (n=81), which included those who underwent liver biopsy (n=19) and those treated with corticosteroids (n=22), was relatively small for identifying prognostic factors. Corticosteroids are the recommended treatment for patients with severe AH [1]. In this study, corticosteroids were used in only 27% of patients, even though the included patients all had severe AH. Although corticosteroids are not absolutely contraindicated, they are not frequently used in the setting of infections, gastrointestinal bleeding, and acute kidney injury. In this study, infections (24%), gastrointestinal bleeding (21%), and acute kidney injury (44%) were frequent, which may explain the low rate of corticosteroid use in this study. However, the exact reason that corticosteroids were or were not used cannot be ascertained because of the retrospective nature of the study. Hence, the findings from our study warrant further evaluation using a larger cohort.
In summary, after the MELD-based allocation system was implemented, DDLT increased in patients with severe AH, which increased overall survival. LT was a significant prognostic factor for overall survival, with a large difference in survival according to LT (88% vs. 44% at 1 year). However, recidivism was also a concern, as there was one case of mortality due to recidivism in this study. The MELD score and corticosteroid response were factors associated with TFS and, therefore, could be used to help predict patient prognosis and plan management options. Further studies evaluating optimal selection criteria for emergent LT in patients with severe AH are urgently needed in Korea, as the current MELD-based allocation system does not consider the period of abstinence or have criteria to justify the use of precious liver grafts for severe AH patients.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: DHS. Data curation: TJK. Formal analysis: TJK, DHS. Writing–original draft: TJK, DHS. Writing– review & editing: DHS, WK, GYG, YHP, MSC, JHL, KCK, SWP.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.4285/kjt.20.0054.
REFERENCES
1. Korean Association for the Study of the Liver (KASL). 2013; KASL clinical practice guidelines: management of alcoholic liver disease. Clin Mol Hepatol. 19:216–54. DOI: 10.3350/cmh.2013.19.3.216. PMID: 24133661. PMCID: PMC3796673.


2. Lucey MR, Mathurin P, Morgan TR. 2009; Alcoholic hepatitis. N Engl J Med. 360:2758–69. DOI: 10.1056/NEJMra0805786. PMID: 19553649.

3. Shipley LC, Kodali S, Singal AK. 2019; Recent updates on alcoholic hepatitis. Dig Liver Dis. 51:761–8. DOI: 10.1016/j.dld.2019.03.023. PMID: 31010745.


4. Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. 2011; Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 60:255–60. DOI: 10.1136/gut.2010.224097. PMID: 20940288.


5. Liu M, Shah VH. 2019; New prospects for medical management of acute alcoholic hepatitis. Clin Liver Dis (Hoboken). 13:131–5. DOI: 10.1002/cld.792. PMID: 31236260. PMCID: PMC6544414.



6. Vergis N, Atkinson SR, Thursz MR. 2019; The future of therapy for alcoholic hepatitis: beyond corticosteroids. J Hepatol. 70:785–7. DOI: 10.1016/j.jhep.2019.01.016. PMID: 30791978. PMCID: PMC6420340.


7. Pavlov CS, Varganova DL, Casazza G, Tsochatzis E, Nikolova D, Gluud C. 2019; Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database Syst Rev. 4:CD001511. DOI: 10.1002/14651858.CD001511.pub4. PMID: 30964545. PMCID: PMC6455893.

8. Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, et al. 2017; In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 152:1068–77. DOI: 10.1053/j.gastro.2016.12.019. PMID: 28043903. PMCID: PMC6381387.


9. Obed A, Bashir A, Stern S, Jarrad A. 2018; Severe acute alcoholic hepatitis and liver transplant: a never-ending mournful story. Clin Mol Hepatol. 24:358–66. DOI: 10.3350/cmh.2018.0044. PMID: 30360030. PMCID: PMC6313024.



10. Haugen CE, Cameron AM. 2020; Early liver transplantation in acute alcoholic hepatitis. Semin Liver Dis. 40:29–33. DOI: 10.1055/s-0039-3399560. PMID: 31711253.


11. Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. 2011; Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 365:1790–800. DOI: 10.1056/NEJMoa1105703. PMID: 22070476.


12. Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, et al. 2018; Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology. 155:422–30. DOI: 10.1053/j.gastro.2018.04.009. PMID: 29655837. PMCID: PMC6460480.



13. Alsahhar JS, Mehta A, Lepe R. 2019; Con: liver transplantation should not be performed in patients with acute alcoholic hepatitis. Clin Liver Dis (Hoboken). 13:144–7. DOI: 10.1002/cld.779. PMID: 31236263. PMCID: PMC6544416.



14. Kubiliun MJ, Rich NE, Singal A, Mufti AR. 2019; Pro: liver transplantation should be considered in select patients with acute alcoholic hepatitis. Clin Liver Dis (Hoboken). 13:140–3. DOI: 10.1002/cld.780. PMID: 31236262. PMCID: PMC6544410.



15. Donckier V, Lucidi V, Gustot T, Moreno C. 2014; Ethical considerations regarding early liver transplantation in patients with severe alcoholic hepatitis not responding to medical therapy. J Hepatol. 60:866–71. DOI: 10.1016/j.jhep.2013.11.015. PMID: 24291238.


16. Daswani R, Kumar A, Sharma P, Singla V, Bansal N, Arora A. 2018; Role of liver transplantation in severe alcoholic hepatitis. Clin Mol Hepatol. 24:43–50. DOI: 10.3350/cmh.2017.0027. PMID: 29316778. PMCID: PMC5875200.



17. Min SI, Ahn C, Han DJ, Kim SI, Chung SY, Lee SK, et al. 2015; To achieve national self-sufficiency: recent progresses in deceased donation in Korea. Transplantation. 99:765–70. DOI: 10.1097/TP.0000000000000412. PMID: 25226175.

18. Kim MS. 2016; Modification of emergency status in deceased donor liver allocation: evidence for Korean Model of End-stage Liver Disease (MELD) system. J Korean Soc Transplant. 30:51–8. DOI: 10.4285/jkstn.2016.30.2.51.

19. Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. 2016; Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 150:785–90. DOI: 10.1053/j.gastro.2016.02.042. PMID: 26921783. PMCID: PMC5287362.



20. Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. 2012; Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 56:2328–35. DOI: 10.1002/hep.25947. PMID: 22806618. PMCID: PMC3492528.



21. Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, et al. 2005; MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 41:353–8. DOI: 10.1002/hep.20503. PMID: 15660383.


22. Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. 2007; The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 45:1348–54. DOI: 10.1002/hep.21607. PMID: 17518367.


23. Shawcross DL, O'Grady JG. 2010; The 6-month abstinence rule in liver transplantation. Lancet. 376:216–7. DOI: 10.1016/S0140-6736(10)60487-4. PMID: 20656110.

24. Brown RS Jr. 2011; Transplantation for alcoholic hepatitis: time to rethink the 6-month "rule". N Engl J Med. 365:1836–8. DOI: 10.1056/NEJMe1110864. PMID: 22070481.

25. Lee BP, Terrault NA. 2018; Early liver transplantation for severe alcoholic hepatitis: moving from controversy to consensus. Curr Opin Organ Transplant. 23:229–36. DOI: 10.1097/MOT.0000000000000507. PMID: 29389821. PMCID: PMC6423506.


26. Chung HG, Sinn DH, Kang W, Choi GS, Kim JM, Joh JW. 2021; Incidence of and risk factors for alcohol relapse after liver transplantation for alcoholic liver disease: comparison between deceased donor and living donor liver transplantation. J Gastrointest Surg. 25:672–80. DOI: 10.1007/s11605-020-04540-7. PMID: 32095927.


27. Lee J, Kim DG, Lee JY, Lee JG, Joo DJ, Kim SI, et al. 2019; Impact of model for end-stage liver disease score-based allocation system in Korea: a nationwide study. Transplantation. 103:2515–22. DOI: 10.1097/TP.0000000000002755. PMID: 30985735.


28. Im GY, Cameron AM, Lucey MR. 2019; Liver transplantation for alcoholic hepatitis. J Hepatol. 70:328–34. DOI: 10.1016/j.jhep.2018.11.007. PMID: 30658734.


29. Lee BP, Samur S, Dalgic OO, Bethea ED, Lucey MR, Weinberg E, et al. 2019; Model to calculate harms and benefits of early vs delayed liver transplantation for patients with alcohol-associated hepatitis. Gastroenterology. 157:472–80. DOI: 10.1053/j.gastro.2019.04.012. PMID: 30998988. PMCID: PMC6650344.



Fig. 1
Change in the proportion of patients receiving liver transplantation (LT) before and after Model for End-Stage Liver Disease (MELD) implementation. LDLT, live donor liver transplant; DDLT, deceased donor liver transplant.
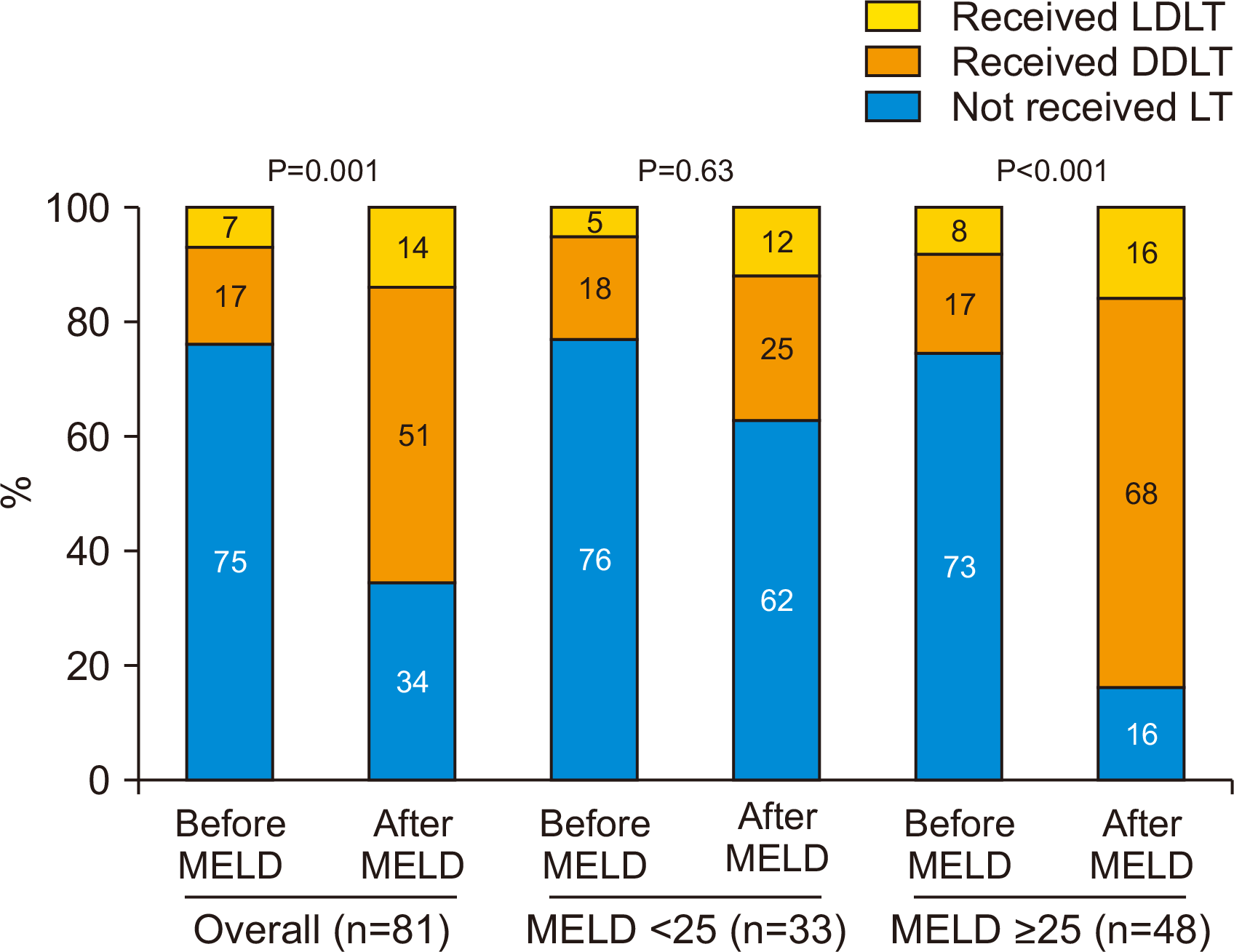
Fig. 2
Flowchart of patients from enrollment concerning corticosteroid use, liver transplantation (LT), and death or survival.
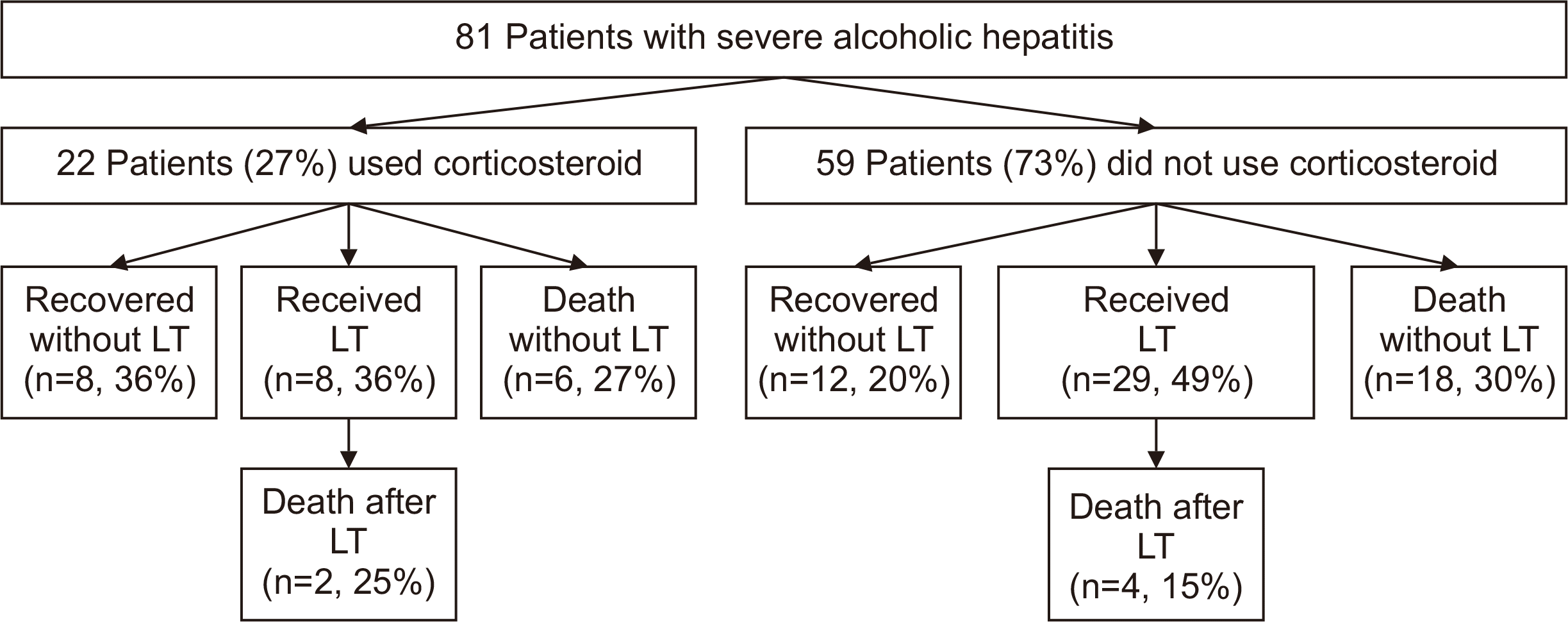
Fig. 3
Kaplan-Meier estimates of overall survival in the eras before and after Model for End-Stage Liver Disease (MELD)-based allocation system implementation. Before MELD: Jan 2014–Jun 2016; After MELD: Jul 2016–Dec 2018.
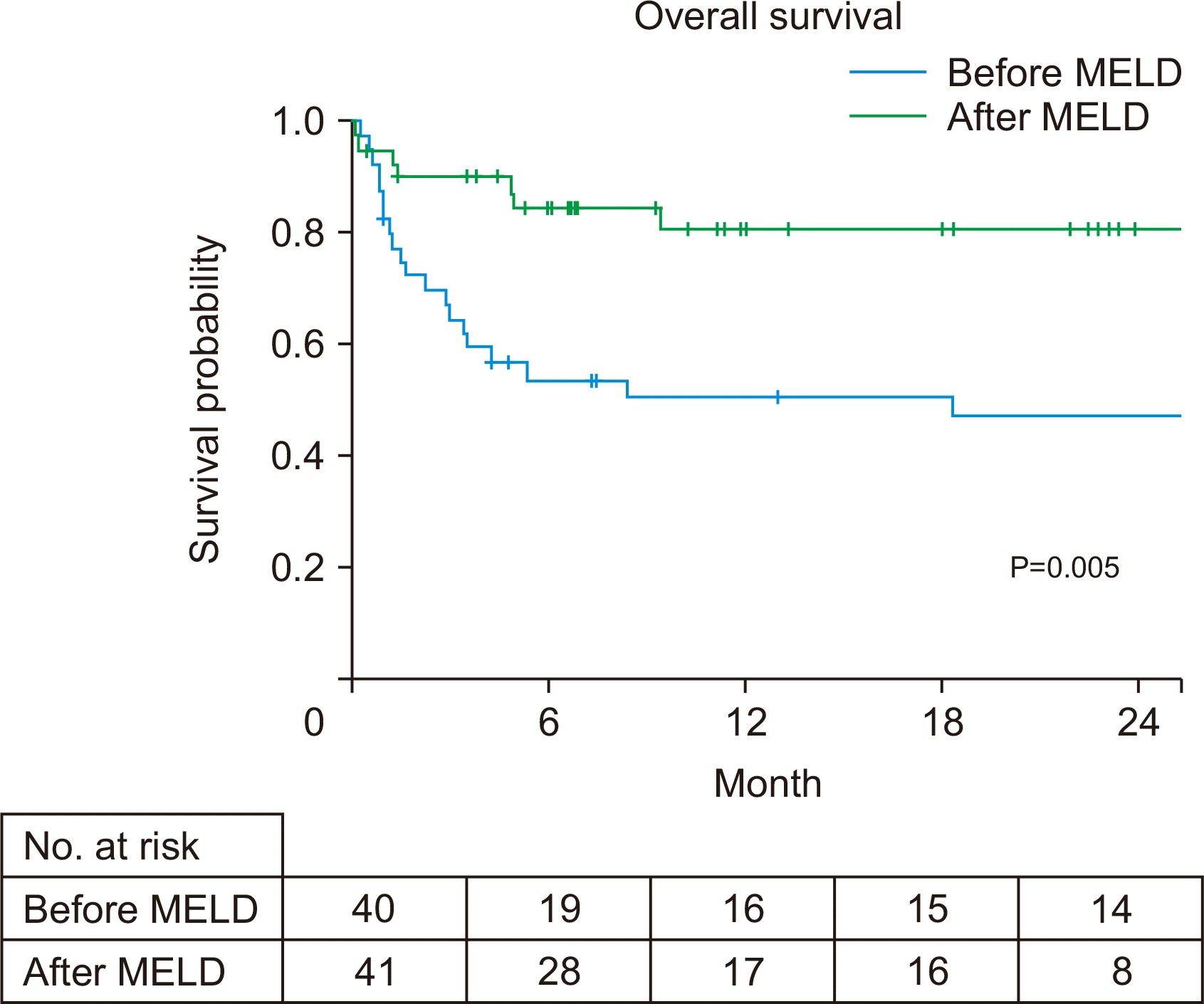
Table 1
Baseline patient characteristics in the eras before and after implementation of the MELD-based allocation system
Table 2
Factors associated with overall survival
Table 3
Factors associated with transplant-free survival




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download