This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Chronic antibody-mediated rejection (CABMR) is an important cause of late graft loss. De novo donor-specific antibody (dnDSA) is an important prognostic factor for long-term allograft outcomes. However, the prognosis of CABMR based on the presence of dnDSA is uncertain.
Methods
We retrospectively analyzed 35 kidney transplant recipients with CABMR between 2010 and 2018. Fourteen recipients had no detectable DSA, and 21 recipients had detectable DSA. We investigated the pathologic findings at diagnosis of CABMR, allograft function 12 months later, related factors for allograft failure, and allograft survival rate based on the presence of dnDSA.
Results
The pathologic findings showed that acute and chronic changes were more severe in the dnDSA (+) group than in the dnDSA (–) group. There was no significant difference in the allograft function 12 months after the diagnosis of CABMR and in the amount of proteinuria at diagnosis between the two groups. However, the death-censored graft survival rate was lower in the high-proteinuria group than in the low-proteinuria group in both groups. The treatment rate of recipients was higher in the dnDSA (+) group than in the dnDSA (–) group; however, there was no significant difference in the death-censored graft survival rate between the two groups.
Conclusions
Although the effect of dnDSA on the prognosis of CABMR is not clear, it would be important not to neglect treatment for CABMR with risk factors for allograft failure even without dnDSA. Continuous and rigorous surveillance of DSA and allograft function is needed in patients with CABMR.
Go to :

Keywords: Kidney transplantation, Graft rejection, Antibodies, Risk factor, Treatment
|
HIGHLIGHTS |
We investigated the clinical outcomes of chronic antibody-mediated rejection (CABMR) based on the presence of de novo donor-specific antibody (dnDSA). Pathologic findings showed that acute and chronic change was more severe in the dnDSA (+) group than in the dnDSA (–) group. The treatment rate of recipients was higher in the dnDSA (+) group than in the dnDSA (–) group, but, there was no difference of prognosis between the two groups. Continuous and rigorous surveillance of DSA and allograft function is needed in patients with CABMR.
|
Go to :

INTRODUCTION
Chronic antibody-mediated rejection (CABMR) is an important cause of late graft loss. CABMR is defined by the Banff classification as follows: (1) morphologic evidence of chronic tissue injury, (2) evidence of current or recent antibody interaction with the vascular endothelium, and (3) serologic evidence of donor-specific antibodies (DSAs) to the human leukocyte antigen (HLA) or other antigens [
1]. Since CABMR is known to be an extension of the acute antibody-mediated rejection that is not treated, pathologic findings are very important for the differentiation, diagnosis, and prediction of prognosis [
2,
3]. Also, the presence of DSA during the diagnosis of CABMR is emphasized in the Banff classification [
4,
5]. Recently, it has been reported that de novo DSA (dnDSA) is an independent risk factor for allograft failure [
6]. However, the prognosis of CABMR based on the presence of dnDSA is uncertain. Therefore, we investigated the clinical outcomes of CABMR based on the presence of dnDSA.
Go to :

METHODS
Human and Animal Rights
We conducted this study in compliance with the principles of the Declaration of Helsinki. The study protocol was reviewed by the Institutional Review Board of Keimyung University Dongsan Medical Center, and they approved this study (IRB No. 2018-12-033). The Institutional Review Board waived the requirement for informed consent because the patients’ data were used retrospectively for research, except for important personal information, which the individual was identified by, and was explained to all the donors’ families and all recipients before kidney transplant (KT). Therefore, this study did not contain any identifiable personal information, except for the clinical process and outcome as a retrospective medical record study.
Study Design
We retrospectively analyzed 35 kidney transplant recipients (KTRs) diagnosed with CABMR between 2010 and 2018. We excluded KTRs with positive crossmatching prior to KT, ABO-incompatible KT, and those whose data were insufficient. We divided the study population into two groups as follows: 14 KTRs without and 21 KTRs with dnDSA. We defined KTRs without dnDSA as KTRs with undetectable donor-specific anti-HLA antibodies and KTRs with dnDSA as KTRs with detectable donor-specific anti-HLA antibodies. We investigated the baseline characteristics of the study population, pathologic findings at the time of diagnosis of CABMR, change in allograft function before the diagnosis, and at 1, 3, 6, and 12 months after the diagnosis, the amount of proteinuria at the time of diagnosis, response to treatment for CABMR, and allograft survival rate based on the presence of dnDSA.
Immunosuppression Protocols
We used basiliximab (20 mg on days 0 and 4, Simulect; Novartis, Basel, Switzerland) for KTRs with low immunologic risk and anti-thymocyte globulin (thymoglobulin; Sanofi Genzyme, Cambridge, MA, USA; 1.5 mg/kg on day 0 and 1.0 mg/kg on days 1 through 3) for KTRs with high immunologic risk as immunosuppressants for induction treatment. We used cyclosporine (3 mg/kg, twice a day; Sandimmun; Novartis) or tacrolimus (0.05 mg/kg, twice a day, Prograf; Astellas Pharma Inc., Toyama, Japan), prednisolone (30 mg, once a day), and mycophenolate mofetil (750 or 1,000 mg, twice a day, CellCept; Hoffmann-La Roche Inc., Nutley, NJ, USA) as immunosuppressants for maintenance therapy. The treatment protocol for CABMR was as follows: high-dose intravenous immunoglobulin (IVIG; 2 g/kg) after a single dose of rituximab (375 mg/m2) infusion, and treatment with (2–3 sessions) or without plasmapheresis according to the pathologic findings, allograft function, and willingness to undergo the therapy.
Demographic and Clinical Data
We investigated the age of donors and recipients at the time of CABMR diagnosis, sex of the donors and recipients, KT type, frequency of KT, dialysis type prior to KT, dialysis vintage, causes of end-stage renal disease, the number of HLA mismatches, immunosuppressants for induction and maintenance treatment, previous biopsy-proven acute rejection (BPAR), the amount of proteinuria at the time of diagnosis of CABMR, panel reactive antibody (PRA) class I or II >50%, positive DSA class I or II, mean fluorescence index (MFI) value, and pathologic findings. Allograft protocol biopsies were performed at 12 months after KT, and indication biopsies were performed at the time of allograft dysfunction or if the KTR had persistent proteinuria. All BPARs were the result of an indication biopsy in this study. Allograft biopsies were analyzed using the Banff 2017 classification, which defined CABMR as follows: (1) morphologic evidence of chronic tissue injury, (2) evidence of current/recent antibody interaction with the vascular endothelium, and (3) serologic evidence of DSAs to the HLA or other antigens [
1]. Allograft function was measured as the estimated glomerular filtration rate (eGFR) based on the modification of diet in the renal disease (MDRD) formula before the diagnosis of CABMR, at the time of diagnosis of CABMR, and at 1, 3, 6, and 12 months after diagnosis or treatment. Proteinuria was measured using the spot urine protein-creatinine ratio. We examined PRA screening and identification (class I and class II) before KT and annually after KT to evaluate KTRs with high immunological risks. We defined KTRs with PRA >50% as high-risk immunological patients. DSA was analyzed with a Luminex Single Antigen assay, using LABscreen Single Antigen HLA class I and class II (One Lambda, Canoga Park, CA, USA) according to the manufacturer’s manual prior to KT and at 1, 3, 6, and 12 months and annually after KT or at the time of diagnosis and monthly after the diagnosis or treatment of CABMR. We defined DSA that existed before KT as preformed DSA, and when DSA occurred newly during the follow-up period after KT without preformed DSA or was different from preformed DSA was defined as dnDSA.
Statistical Analysis
Continuous variables were analyzed using the Mann–Whitney U-test, and categorical variables were analyzed by the chi-square or Fisher’s exact test. Graft and patient survival rates were evaluated using the Kaplan-Meier analysis with the log-rank test. Univariate and multivariate analyses with Cox regression analysis were performed to investigate the risk factors for allograft failure. P-values <0.05. were considered statistically significant. Statistical analysis was performed using SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
Go to :

RESULTS
Baseline Characteristics of the Study Population
The mean age of KTRs at the time of diagnosis of CABMR was 50±13 years, and 24 patients (68.6%) were male. Living donor KT occurred in 24 patients (68.6%), and 30 patients (85.7%) were the first KT recipients. All patients underwent hemodialysis before KT, and 21 patients (77.1%) had chronic glomerulonephritis as the primary renal disease. The main immunosuppressants used were tacrolimus (30, 85.7%) and cyclosporine (5, 14.3%). Eight patients (22.9%) experienced BPAR prior to the diagnosis of CABMR. Median proteinuria at the time of diagnosis of CABMR was 1.1 g (interquartile range [IQR], 0.3–2.8 g). The time from KT to the development of dnDSA was 91.6±77.4 months. The time until the diagnosis of CABMR after transplantation was 74.9 months (IQR, 42.8–142.6 months).
Comparison of the Clinical and Laboratory Parameters Based on the Presence of Detectable dnDSA
The mean follow-up duration was 129.0±72.7 months. There were no significant differences in the mean age of donors and recipients, the proportion of sex distribution, KT type, Kidney Donor Profile Index score, KT number, duration of dialysis, dialysis type before KT, causes of end-stage renal disease, the number of HLA mismatches, induction and maintenance immunosuppressants, the rate of PRA >50%, and preformed DSA positivity, and the rate of previous BPAR between the dnDSA (–) and dnDSA (+) groups. There was one case (7.1%) of T-cell mediated rejection (TCMR) in the undetectable dnDSA group and one case (4.8%) of TCMR in the detectable dnDSA group. There were no significant differences in the coexistence of TCMR between the two groups. The time from KT until the diagnosis of CABMR showed no significant difference between the two groups (
Table 1).
Table 1
Comparison of clinical and laboratory parameters based on detectable de novo DSA
|
Variable |
Undetectable
de novo DSA (n=14) |
Detectable
de novo DSA (n=21) |
P-value |
|
Recipient age at diagnosis (yr) |
49.0±11.7 |
50.6±13.4 |
0.723 |
|
Recipient sex (male:female) |
8 (57):6 (43) |
16 (76):5 (24) |
0.283 |
|
Donor age at diagnosis (yr) |
44.1±11.7 |
43.5±14.6 |
0.907 |
|
Donor sex (male:female) |
7 (50):7 (50) |
10 (48):11 (52) |
1.000 |
|
KDPI score (%) |
53.5±23.3 |
82.3±17.7 |
0.208 |
|
Dialysis duration (mo) |
41.6±48.9 |
35.6±44.1 |
0.706 |
|
Donor type (living:deceased) |
9 (64):5 (36) |
15 (71):6 (29) |
0.721 |
|
Cause of end-stage renal disease |
|
|
0.141 |
|
Glomerulonephritis |
10 (72) |
17 (81) |
|
|
Hypertension |
1 (7) |
4 (19) |
|
|
Diabetes mellitus |
1 (7) |
0 |
|
|
Polycystic kidney disease |
2 (14) |
0 |
|
|
HLA mismatch number |
3.5±1.7 |
3.7±0.9 |
0.600 |
|
Preformed DSA |
0 |
4 (19.0) |
0.133 |
|
PRA >50% at diagnosis of CABMR |
3 (21.4) |
14 (66.7) |
0.032 |
|
Class I DSA (A:B) at diagnosis of CABMR |
NA |
3:3 |
|
|
Class II DSA (DR:DQ) at diagnosis of CABMR |
NA |
11:7 |
|
|
Induction |
|
|
0.532 |
|
Basiliximab |
7 (50.0) |
13 (61.9) |
|
|
Antithymocyte globulin |
1 (7.1) |
3 (14.3) |
|
|
None |
6 (42.9) |
5 (23.8) |
|
|
Main immunosuppressant |
|
|
|
|
Tacrolimus:cyclosporine |
|
|
|
|
At KT |
11 (78.6):3 (21.4) |
18 (85.7):3 (14.3) |
0.664 |
|
At diagnosis |
12 (85.7):2 (14.3) |
18 (85.7):3 (14.3) |
1.000 |
|
After diagnosis or treatment |
12 (85.7):2 (14.3) |
17 (81.0):4 (19.0) |
0.642 |
|
Previous BPAR |
3 (21.4) |
5 (23.8) |
1.000 |
|
Coexistence of TCMR |
1 (7.1) |
1 (4.8) |
1.000 |
|
Time from KT to development of de novo DSA (mo) |
NA |
91.6±77.4 |
|
|
Time from KT to diagnosis of CABMR (mo) |
101.6±59.6 |
90.3±72.9 |
0.634 |

Comparison of the Pathologic Findings of Allograft, Allograft Function, Proteinuria, and Treatment Options Based on Detectable dnDSA
Pathologic findings showed that acute changes such as glomerulitis, peritubular capillaritis, microvascular inflammation, arteritis, and tubulitis were more severe in the dnDSA (+) group than in the dnDSA (–) group; however, there was no significant difference between the two groups. Chronic changes such as transplant glomerulopathy, arterial intimal fibrosis, and interstitial fibrosis/tubular atrophy were also not significantly different between the two groups. The proportion of C4d positivity was not significantly different between the two groups (
Table 2).
Table 2
Comparison of pathologic findings and clinical outcomes based on detectable de novo DSA
|
Variable |
Undetectable
de novo DSA (n=14) |
Detectable
de novo DSA (n=21) |
P-value |
|
Glomerulitis (g score >1) |
6 (46.2) |
15 (71.4) |
0.168 |
|
Peritubular capillaritis (ptc score >1) |
8 (57.1) |
17 (81.0) |
0.151 |
|
Microvascular inflammation (g+ptc score >1) |
11 (78.6) |
20 (95.2) |
0.279 |
|
Arteritis (v score >0) |
2 (14.3) |
5 (23.8) |
0.676 |
|
Tubulitis (t score ≥1) |
11 (78.6) |
16 (76.2) |
1.000 |
|
Transplant glomerulopathy (cg score ≥1) |
9 (69.2) |
18 (85.7) |
0.387 |
|
Arterial intimal fibrosis (cv score >1) |
3 (21.4) |
7 (33.3) |
0.704 |
|
IF/TA (ci+ct scores) |
|
|
1.000 |
|
2–3 |
6 (42.9) |
10 (47.6) |
|
|
≥4 |
8 (57.1) |
11 (52.4) |
|
|
Positive C4d |
6 (42.9) |
10 (47.6) |
1.000 |
|
Allograft function |
|
|
|
|
MDRD eGFR (mL/min/1.73 m2) |
|
|
|
|
1 Month before diagnosis |
43.8±10.5 |
45.7±13.5 |
0.654 |
|
At diagnosis |
34.3±9.5 |
38.8±12.3 |
0.261 |
|
1 Month after diagnosis or treatment |
33.6±10.6 |
44.1±18.4 |
0.043 |
|
3 Months after diagnosis or treatment |
31.3±11.2 |
44.9±18.3 |
0.092 |
|
6 Months after diagnosis or treatment |
29.3±10.6 |
39.9±16.4 |
0.033 |
|
12 Months after diagnosis or treatment |
27.4±11.7 |
36.9±19.8 |
0.174 |
|
Proteinuria at diagnosis (g/day) |
1.7 (0.4–5.4) |
1.1 (0.3–2.2) |
0.207 |
|
Proteinuria (≥1.5 g/day) at diagnosis |
7 (50.0) |
6 (28.6) |
0.288 |
|
Rituximab+IVIG |
3 (21.4) |
10 (47.6) |
0.162 |

There was no significant difference in the allograft function at 12 months after the diagnosis of CABMR and the difference in the amount of proteinuria at diagnosis between the dnDSA (–) and dnDSA (+) groups. The use of rituximab and IVIG treatment was higher in the dnDSA (+) group than in the dnDSA (–) group (
Table 2).
Comparison of Death-Censored Allograft Survival and Risk Factors for Graft Failure Based on Detectable dnDSA
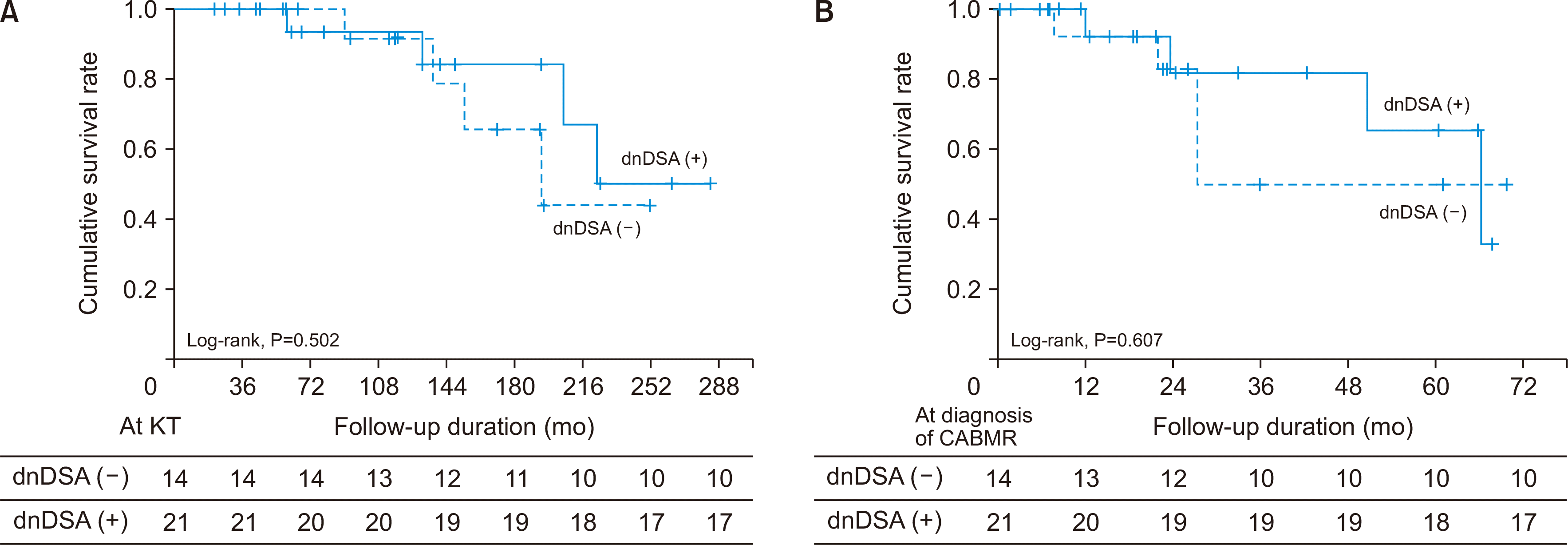
Nine patients (25.7%) developed graft failure, including 5 (35.7%) patients in the dnDSA (–) group and 4 (19.0%) in the dnDSA (+) group. The causes of graft failure were as follows: chronic rejection, 4 (28.6%) and 3 (7.1%); infection, 1 (7.1%) and 1 (4.8%) in the dnDSA (–) and dnDSA (+) groups, respectively. In the Kaplan-Meier analysis, there was no significant difference in the death-censored overall graft survival rate (
Fig. 1A) and death-censored graft survival rate after the diagnosis of CABMR (
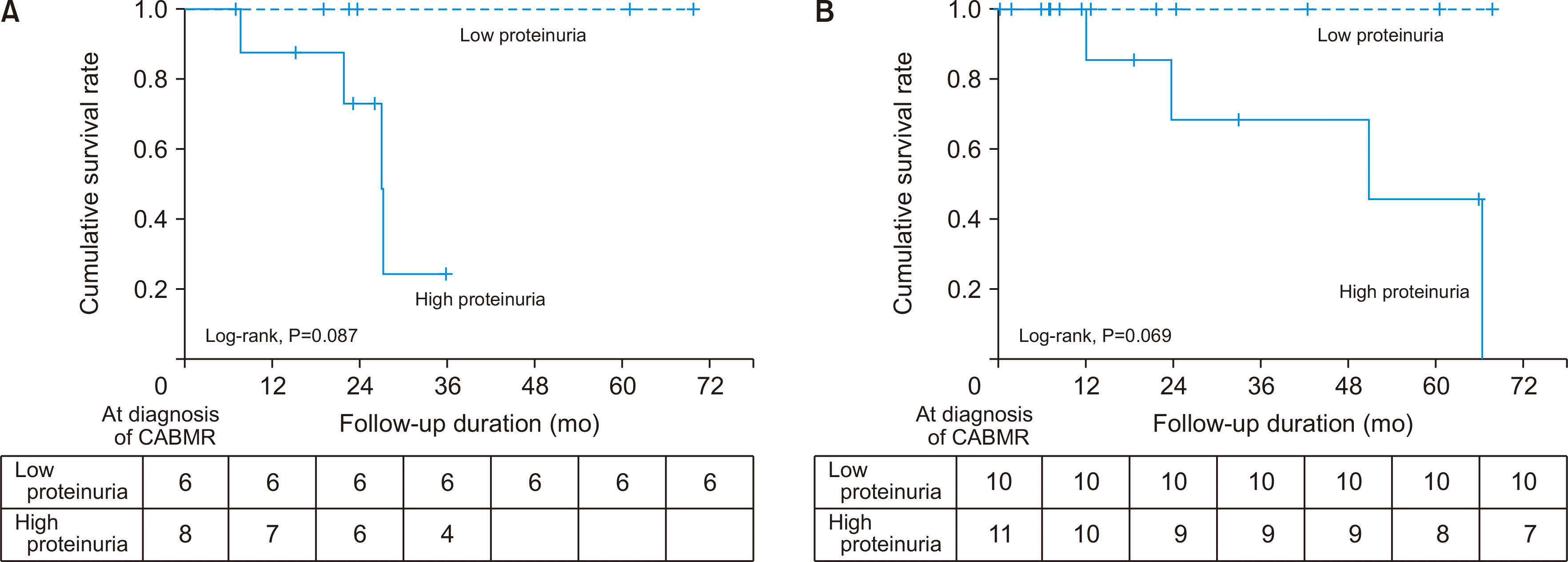
Fig. 1B) between the dnDSA (–) and dnDSA (+) groups. In the subgroup analysis, the death-censored graft survival rate was lower in the high-proteinuria group than in the low-proteinuria group in both the dnDSA (–) and dnDSA (+) groups (
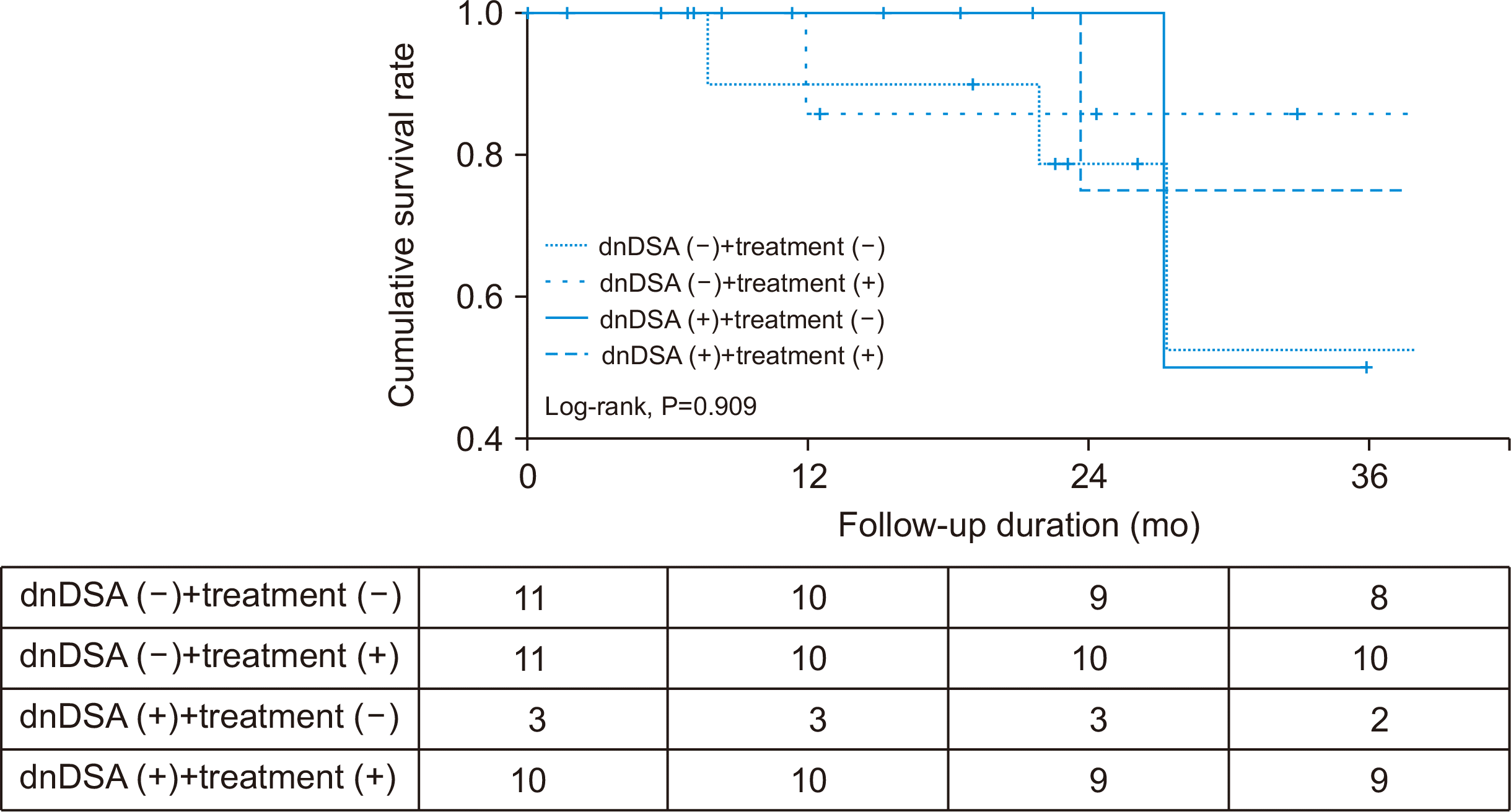
Fig. 2). However, there was no significant difference in the death-censored graft survival rate between the two groups, regardless of the treatment administered (
Fig. 3). On multivariate Cox regression analysis, deceased donor kidney transplantation and eGFR at 12 months after the diagnosis of CABMR were the risk factors associated with graft failure in KT after adjusting for the significant variables in the univariate analysis (hazard ratio, 57.013; 95% confidence interval, 1.698–1,914.123, P=0.024; hazard ratio, 0.850; 95% confidence interval, 0.738–0.980, P=0.025) (
Table 3).
 | Fig. 1Comparison of death-censored overall graft survival rate (A) and death-censored graft survival rate after diagnosis of chronic antibody-mediated rejection (CABMR) (B) according to the presence of de novo donor-specific antibody (dnDSA). 
|
 | Fig. 2Comparison of death-censored graft survival rate between the high proteinuria and low proteinuria in the dnDSA (–) group (A) and dnDSA (+) group (B). CABMR, chronic active antibody-mediated rejection; dnDSA, de novo donor-specific antibody. 
|
 | Fig. 3Comparison of death-censored graft survival rate among DSA (–)+treatment (–), DSA (–)+treatment (+), DSA (+)+treatment (–), and DSA (+)+treatment (+). dnDSA, de novo donor-specific antibody. 
|
Table 3
Risk factors associated with graft failure in kidney transplant recipients with CABMR
|
Variable |
Univariate |
|
Multivariate |
|
|
|
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
|
Recipient age |
0.999 |
0.935–1.067 |
0.971 |
|
|
|
|
|
Recipient male sex |
0.723 |
0.169–3.096 |
0.662 |
|
|
|
|
|
Donor age |
1.009 |
0.950–1.070 |
0.777 |
|
|
|
|
|
Donor male sex |
1.281 |
0.339–4.849 |
0.715 |
|
|
|
|
|
Deceased donor kidney transplantation |
6.542 |
1.246–34.359 |
0.026 |
|
57.013 |
1.698–1,914.123 |
0.024 |
|
Thymoglobulin induction |
1.656 |
0.175–15.707 |
0.660 |
|
|
|
|
|
Previous acute rejection |
0.427 |
0.053–3.450 |
0.425 |
|
|
|
|
|
HLA mismatches |
0.640 |
0.385–1.065 |
0.086 |
|
|
|
|
|
PRA >50% at diagnosis of CABMR |
0.791 |
0.186–3.359 |
0.751 |
|
|
|
|
|
DSA positivity at diagnosis of CABMR |
0.511 |
0.136–1.925 |
0.321 |
|
8.893 |
0.492–160.725 |
0.139 |
|
eGFR at 12 months after diagnosis of CABMR |
0.893 |
0.800–0.995 |
0.043 |
|
0.850 |
0.738–0.980 |
0.025 |
|
Proteinuria >1.5 g/day |
13.912 |
1.732–111.711 |
0.013 |
|
3.355 |
0.132–85.048 |
0.463 |
|
Rituximab and IVIG |
1.730 |
0.430–6.969 |
0.441 |
|
0.259 |
0.022–2.984 |
0.279 |

Five patients (14.3%) died, including one (7.1%) patient in the dnDSA (–) group and 4 (19.0%) in the dnDSA (+) group. The causes of death were as follows: infection, 1 (7.1%) and 3 (14.3%); alveolar hemorrhage, 0 and 1 (4.8%) in the dnDSA (–) and dnDSA (+) groups, respectively. In the Kaplan-Meier analysis, patient survival rates showed no significant differences between the dnDSA (–) and dnDSA (+) groups (
Table 3).
Go to :

DISCUSSION
Our study found that the allograft outcome in the dnDSA (+) group was similar to that of the dnDSA (–) group. Both groups showed low graft and patient survival rates. Furthermore, the pathologic findings did not differ between the two groups. In particular, the Banff 2013 classification expressed negative DSA CABMR as suspicious CABMR [
7]; however, in the Banff 2017 classification, the presence of DSA was imposed, and when there was no DSA, C4d was also supported for the diagnosis of CABMR [
4]. However, our study showed that there was no significant difference in the allograft outcome between the C4d (+) and C4d (–) subgroups in the dnDSA (–) or dnDSA (+) groups. In the subgroup analysis, we compared the clinicopathologic parameters according to graft failure, and there were no significant differences regardless of the presence of dnDSA (data not shown).
The most common treatment options for CABMR patients are rituximab and IVIG, but their effectiveness is still controversial [
8-
10]. Some studies have shown that bortezomib is effective for antibody-mediated rejection [
11]. For the treatment of CABMR, we used the same protocol, irrespective of the presence of dnDSA or C4d, unlike in previous studies, which treated the dnDSA (–) group less aggressively than the dnDSA (+) group [
12]. Furthermore, the treatment rate of recipients was higher in the dnDSA (+) group than in the dnDSA (–) group. However, there was no significant difference in the death-censored graft survival rate between the two groups, regardless of the treatment. Although it has been reported that the combination treatment of rituximab and IVIG might be effective for CABMR, the results of our study showed that this is questionable regarding the presence of dnDSA. We also investigated changes in dnDSA after treatment. In our research, 21 KTRs had dnDSA for the diagnosis of CABMR. Among them, 10 KTRs received treatment with CABMR. The MFI values of six KTRs were reduced after treatment, but dnDSA did not result in negative conversion during the follow-up period. Six KTRs had more than 5,000 MFI values or DQ DSA. However, this was not statistically significant because of the low sample size. Larger scale research and long-term follow-up periods are needed to evaluate this outcome.
It is well known that proteinuria is an important prognostic factor in allograft kidney as well as native kidney disease. Ban et al. [
8] reported that proteinuria affects the prognosis of CABMR, which is consistent with the findings of our study. Moreover, in our research, the prognosis of the allograft kidney was found to be related more to the amount of proteinuria than the presence of dnDSA. In other words, the death-censored graft survival rate was lower in the high-proteinuria group than in the low-proteinuria group in both the dnDSA (–) group and dnDSA (+) groups. Furthermore, although there was no significant difference in allograft function within 12 months after the diagnosis of CABMR between the dnDSA (–) and dnDSA (+) groups, the eGFR at 12 months after the diagnosis of CABMR was the risk factor associated with graft failure, regardless of the presence or absence of dnDSA. Therefore, we should aggressively control the allograft function after the development of CABMR.
There are some limitations to our study. First, our study was retrospective, and the sample size was too small to confirm the impact of dnDSA on allograft outcome. Second, follow-up allograft biopsies were not performed. Third, we did not consider and investigate non-HLA DSA in CABMR patients with dnDSA (–).
In conclusion, although the effect of dnDSA on the prognosis of CABMR is not clear, it would be important not to neglect treatment for CABMR even without dnDSA in the case of risk factors such as heavy proteinuria, low allograft function, and deceased donor KT. Therefore, continuous and rigorous surveillance of DSA and allograft function is required in CABMR patients with risk factors.
Go to :

ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by the First Research Support Project of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT in 2018 (NRF-2017R1C1B5076739).
This study was supported by research grant from the Korean Society for Transplantation (2021-00-01002-005).
Author Contributions
Conceptualization: SH. Data curation: YK, JHP. Formal analysis: WYP. Funding acquisition: WYP. Investigation: KJ. Methodology: YK. Project administration: WYP, KJ. Visualization: JHP. Writing–original draft: WYP. Writing–review & editing: WYP, SH.
Go to :

REFERENCES
1. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. 2018; The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 18:293–307. DOI:
10.1111/ajt.14625. PMID:
29243394. PMCID:
PMC5817248.



2. Singh N, Pirsch J, Samaniego M. 2009; Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando). 23:34–46. DOI:
10.1016/j.trre.2008.08.004. PMID:
19027615.


3. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. 2007; Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 53:766–72. DOI:
10.1373/clinchem.2006.077180. PMID:
17332152.


4. Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. 2018; A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 102:1795–814. DOI:
10.1097/TP.0000000000002366. PMID:
30028786. PMCID:
PMC7597974.

5. Solar-Cafaggi D, Marino L, Uribe-Uribe N, Morales-Buenrostro LE. 2018; Antibody-mediated rejection in the Banff classifications of 2007 and 2017: a comparison of renal graft loss prediction capability. Transpl Immunol. 51:40–4. DOI:
10.1016/j.trim.2018.08.008. PMID:
30170180.


6. Wan SS, Chadban SJ, Watson N, Wyburn K. 2020; Development and outcomes of de novo donor-specific antibodies in low, moderate, and high immunological risk kidney transplant recipients. Am J Transplant. 20:1351–64. DOI:
10.1111/ajt.15754. PMID:
31867849.


7. Haas M. 2016; The Revised (2013) Banff Classification for Antibody-Mediated Rejection of Renal Allografts: update, difficulties, and future considerations. Am J Transplant. 16:1352–7. DOI:
10.1111/ajt.13661. PMID:
26696524.


8. Ban TH, Yu JH, Chung BH, Choi BS, Park CW, Kim YS, et al. 2017; Clinical outcome of rituximab and intravenous immunoglobulin combination therapy in kidney transplant recipients with chronic active antibody-mediated rejection. Ann Transplant. 22:468–74. DOI:
10.12659/AOT.903499. PMID:
28775248.


9. Hassan R, Gheith O. 2014; Chronic antibody-mediated rejection: review of literature. Iran J Kidney Dis. 8:93–103. PMID:
24685730.

10. Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, et al. 2018; Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant. 18:927–35. DOI:
10.1111/ajt.14520. PMID:
28949089.


11. Gang S, Han A, Min S, Ha J, Yang J. 2019; Successful treatment of early acute antibody-mediated rejection in an human leukocyte antigen-incompatible and ABO-incompatible living-donor kidney transplant patient. Korean J Transplant. 33:153–8. DOI:
10.4285/jkstn.2019.33.4.153.

Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download