1. Bhatnagar A, Maziak W, Eissenberg T, Ward KD, Thurston G, King BA, Sutfin EL, Cobb CO, Griffiths M, Goldstein LB, Rezk-Hanna M. 2019; Water pipe (hookah) smoking and cardiovascular disease risk: a scientific statement From the American Heart Association. Circulation. 139:e917–36. DOI:
10.1161/CIR.0000000000000671. PMCID:
PMC6600812. PMID:
30845826.

2. WHO Study Group on Tobacco Product Regulation (TobReg). 2015. Waterpipe tobacco smoking: health effects, research needs and recommended actions for regulators. 2nd ed. WHO;Geneva:
3. Jukema JB, Bagnasco DE, Jukema RA. 2014; Waterpipe smoking: not necessarily less hazardous than cigarette smoking: possible consequences for (cardiovascular) disease. Neth Heart J. 22:91–9. DOI:
10.1007/s12471-013-0501-0. PMID:
24307377. PMCID:
PMC3931860.

4. Kaplan B, Sussan T, Rule A, Moon K, Grau-Perez M, Olmedo P, Chen R, Carkoglu A, Levshin V, Wang L, Watson C, Blount B, Calafat AM, Jarrett J, Caldwell K, Wang Y, Breysse P, Strickland P, Cohen J, Biswal S, Navas-Acien A. 2019; Waterpipe tobacco smoke: characterization of toxicants and exposure biomarkers in a cross-sectional study of waterpipe employees. Environ Int. 127:495–502. DOI:
10.1016/j.envint.2019.03.074. PMID:
30981020. PMCID:
PMC6513716.



5. Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA. 2015; Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control. 24(Suppl 1):i22–30. DOI:
10.1136/tobaccocontrol-2014-051907. PMID:
25666550. PMCID:
PMC4345918.

6. Pryor WA, Stone K, Zang LY, Bermúdez E. 1998; Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 11:441–8. DOI:
10.1021/tx970159y. PMID:
9585474.


7. Alsaad AM, Al-Arifi MN, Maayah ZH, Attafi IM, Alanazi FE, Belali OM, Alhoshani A, Asiri YA, Korashy HM. 2019; Genotoxic impact of long-term cigarette and waterpipe smoking on DNA damage and oxidative stress in healthy subjects. Toxicol Mech Methods. 29:119–27. DOI:
10.1080/15376516.2018.1528650. PMID:
30273082.


8. Hadidi KA, Mohammed FI. 2004; Nicotine content in tobacco used in hubble-bubble smoking. Saudi Med J. 25:912–7. PMID:
15235699.

9. Chaouachi K. 2009; Hookah (Shisha, Narghile) smoking and environmental tobacco smoke (ETS). A critical review of the relevant literature and the public health consequences. Int J Environ Res Public Health. 6:798–843. DOI:
10.3390/ijerph6020798. PMID:
19440416. PMCID:
PMC2672364.



10. Mamtani R, Cheema S, Sheikh J, Al Mulla A, Lowenfels A, Maisonneuve P. 2017; Cancer risk in waterpipe smokers: a meta-analysis. Int J Public Health. 62:73–83. DOI:
10.1007/s00038-016-0856-2. PMID:
27421466. PMCID:
PMC5288449.


11. Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. 2015; Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 20:21138–56. DOI:
10.3390/molecules201219753. PMID:
26633317. PMCID:
PMC6331972.



12. Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. 2018; Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol. 9:1162. DOI:
10.3389/fphar.2018.01162. PMID:
30405405. PMCID:
PMC6204759.



13. Bengmark S. 2006; Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 30:45–51. DOI:
10.1177/014860710603000145. PMID:
16387899.

14. Oyagbemi AA, Saba AB, Ibraheem AO. 2009; Curcumin: from food spice to cancer prevention. Asian Pac J Cancer Prev. 10:963–7. PMID:
20192567.

15. Bisht K, Wagner KH, Bulmer AC. 2010; Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 278:88–100. DOI:
10.1016/j.tox.2009.11.008. PMID:
19903510.


16. Kalpana C, Sudheer AR, Rajasekharan KN, Menon VP. 2007; Comparative effects of curcumin and its synthetic analogue on tissue lipid peroxidation and antioxidant status during nicotine-induced toxicity. Singapore Med J. 48:124–30. PMID:
17304391.

17. Stojković D, Petrović J, Soković M, Glamočlija J, Kukić-Marković J, Petrović S. 2013; In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J Sci Food Agric. 93:3205–8. DOI:
10.1002/jsfa.6156. PMID:
23553578.

18. Khan FA, Maalik A, Murtaza G. 2016; Inhibitory mechanism against oxidative stress of caffeic acid. J Food Drug Anal. 24:695–702. DOI:
10.1016/j.jfda.2016.05.003. PMID:
28911606.


19. Akomolafe SF, Akinyemi AJ, Oboh G, Oyeleye SI, Ajayi OB, Omonisi AE, Owolabi FL, Atoyebi DA, Ige FO, Atoki VA. 2018; Co-administration of caffeine and caffeic acid alters some key enzymes linked with reproductive function in male rats. Andrologia. 50:e12839. DOI:
10.1111/and.12839. PMID:
28737015.

20. Dekanski D, Spremo-Potparević B, Bajić V, Živković L, Topalović D, edojević DN Sr, Lazić V, Nedeljković JM. 2018; Acute toxicity study in mice of orally administrated TiO2 nanoparticles functionalized with caffeic acid. Food Chem Toxicol. 115:42–8. DOI:
10.1016/j.fct.2018.02.064. PMID:
29510221.


21. Li Y, Chen LJ, Jiang F, Yang Y, Wang XX, Zhang Z, Li Z, Li L. 2015; Caffeic acid improves cell viability and protects against DNA damage: involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz J Med Biol Res. 48:502–8. DOI:
10.1590/1414-431x20143729. PMID:
25831202. PMCID:
PMC4470308.



22. Ahn CB, Je JY, Kim YS, Park SJ, Kim BI. 2017; Induction of Nrf2-mediated phase II detoxifying/antioxidant enzymes
in vitro by chitosan-caffeic acid against hydrogen peroxide-induced hepatotoxicity through JNK/ERK pathway. Mol Cell Biochem. 424:79–86. DOI:
10.1007/s11010-016-2845-4. PMID:
27743232.

23. Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Wojtyczka RD, Buszman E, Stojko J. 2018; Caffeic acid versus caffeic acid phenethyl ester in the treatment of breast cancer MCF-7 cells: migration rate inhibition. Integr Cancer Ther. 17:1247–59. DOI:
10.1177/1534735418801521. PMID:
30246565. PMCID:
PMC6247537.



24. Andrade M, Benfeito S, Soares P, Magalhães e Silva D, Loureiro J, Borges A, Borges F, Simões M. 2015; Fine-tuning of the hydrophobicity of caffeic acid: studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 5:53915–25. DOI:
10.1039/C5RA05840F.

25. Tanida I, Shirasago Y, Suzuki R, Abe R, Wakita T, Hanada K, Fukasawa M. 2015; Inhibitory effects of caffeic acid, a coffee-related organic acid, on the propagation of hepatitis C virus. Jpn J Infect Dis. 68:268–75. DOI:
10.7883/yoken.JJID.2014.309. PMID:
25672401.


26. Zhu Q, Sun Y, Yun X, Ou Y, Zhang W, Li JX. 2014; Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci Rep. 4:4932. DOI:
10.1038/srep04932. PMID:
24816565. PMCID:
PMC4017214.



27. Salem AM, Ragheb AS, Hegazy MGA, Matboli M, Eissa S. 2019; Caffeic acid modulates miR-636 expression in diabetic nephropathy rats. Indian J Clin Biochem. 34:296–303. DOI:
10.1007/s12291-018-0743-0. PMID:
31391719. PMCID:
PMC6660537.


28. Shraideh ZA, Awaida W, Najjar H, Musleh M. 2011; A modified smoking machine for monitoring the effect of tobacco smoke on albino rats. Jordan J Biol Sci. 4:109–12.
29. Khabour OF, Alzoubi KH, Bani-Ahmad M, Dodin A, Eissenberg T, Shihadeh A. 2012; Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal Toxicol. 24:667–75. DOI:
10.3109/08958378.2012.710918. PMID:
22906173. PMCID:
PMC3752682.



30. Al-Sawalha NA, Alzoubi KH, Khabour OF, Alyacoub W, Almahmood Y. 2019; Effect of waterpipe tobacco smoke exposure during lactation on learning and memory of offspring rats: role of oxidative stress. Life Sci. 227:58–63. DOI:
10.1016/j.lfs.2019.04.049. PMID:
31009626.


31. Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Ali BH. 2019; Waterpipe smoke exposure triggers lung injury and functional decline in mice: protective effect of Gum Arabic. Oxid Med Cell Longev. 2019:8526083. DOI:
10.1155/2019/8526083. PMID:
31178975. PMCID:
PMC6501418.

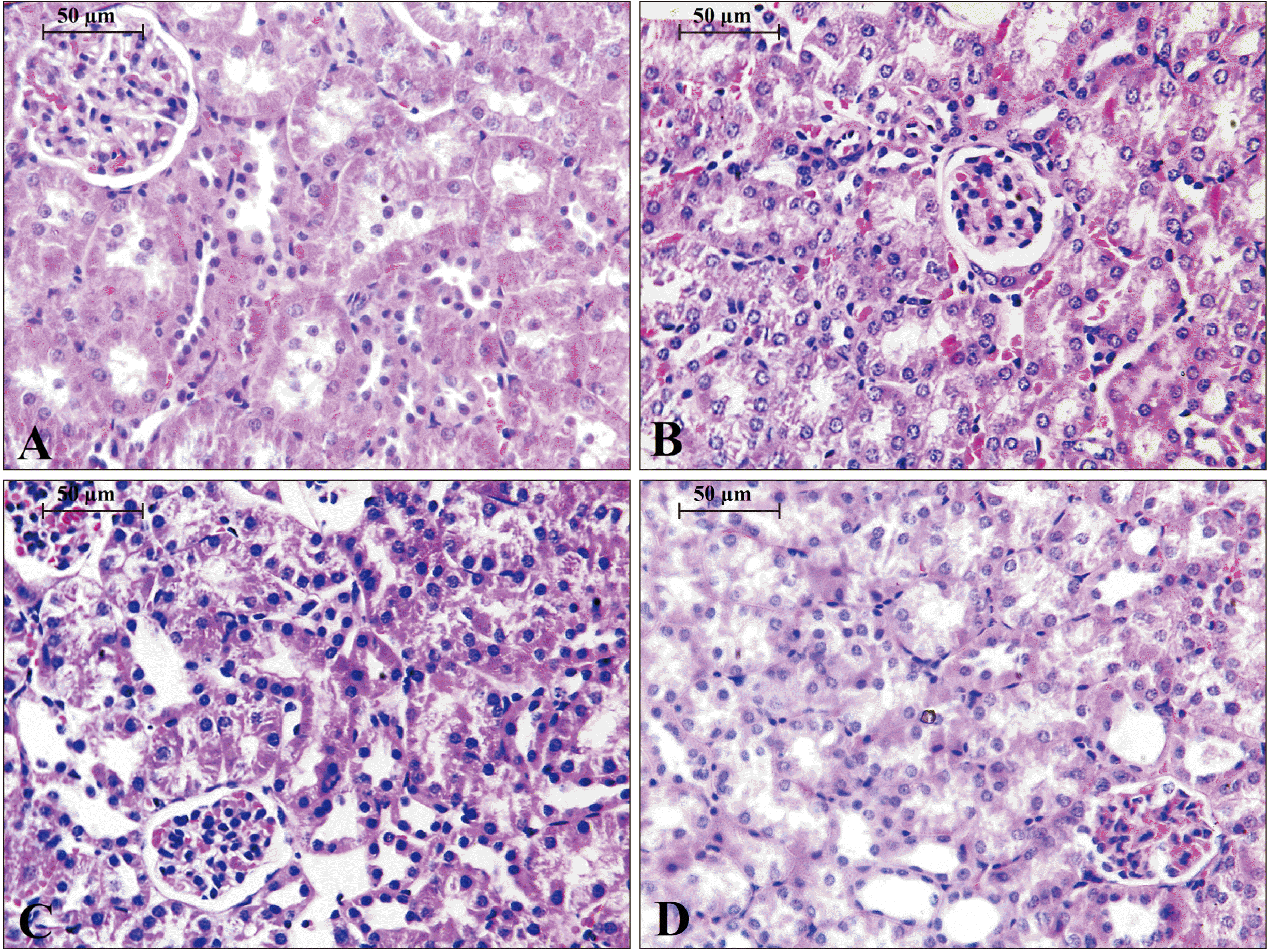
32. Nemmar A, Beegam S, Yuvaraju P, Yasin J, Ali BH, Adeghate E. 2020; Nose-only water-pipe smoke exposure in mice elicits renal histopathological alterations, inflammation, oxidative stress, DNA damage, and apoptosis. Front Physiol. 11:46. DOI:
10.3389/fphys.2020.00046. PMID:
32116758. PMCID:
PMC7026484.



33. Al-Awaida W, Najjar H, Shraideh Z. 2015; Structural characterization of rat ventricular tissue exposed to the smoke of two types of waterpipe. Iran J Basic Med Sci. 18:942–9. PMID:
26730327. PMCID:
PMC4686577.


34. Liang Z, Lu L, Mao J, Li X, Qian H, Xu W. 2017; Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Dis. 8:e3066. DOI:
10.1038/cddis.2017.452. PMID:
28981096. PMCID:
PMC5680574.

35. Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. 2003; Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 63:4375–83. PMID:
12907607.

36. Suzuki M, Betsuyaku T, Ito Y, Nagai K, Odajima N, Moriyama C, Nasuhara Y, Nishimura M. 2009; Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol. 296:L614–23. DOI:
10.1152/ajplung.90443.2008. PMID:
19168576.

37. Janbaz KH, Saeed SA, Gilani AH. 2004; Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 11:424–30. DOI:
10.1016/j.phymed.2003.05.002. PMID:
15330498.


38. Rastogi H, Jana S. 2014; Evaluation of inhibitory effects of caffeic acid and quercetin on human liver cytochrome p450 activities. Phytother Res. 28:1873–8. DOI:
10.1002/ptr.5220. PMID:
25196644.


39. Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. 2008; Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 76:1590–611. DOI:
10.1016/j.bcp.2008.08.008. PMID:
18775680.


40. Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr. 2011; Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 60:409–24. DOI:
10.1007/s00011-011-0308-7. PMID:
21298317.


41. Ghazali WS, Romli AC, Mohamed M. 2017; Effects of honey supplementation on inflammatory markers among chronic smokers: a randomized controlled trial. BMC Complement Altern Med. 17:175. DOI:
10.1186/s12906-017-1703-6. PMID:
28351393. PMCID:
PMC5371194.



42. Petrescu F, Voican SC, Silosi I. 2010; Tumor necrosis factor-alpha serum levels in healthy smokers and nonsmokers. Int J Chron Obstruct Pulmon Dis. 5:217–22. DOI:
10.2147/COPD.S8330. PMID:
20714375. PMCID:
PMC2921689.


43. Mercantepe T, Unal D, Tümkaya L, Yazici ZA. 2018; Protective effects of amifostine, curcumin and caffeic acid phenethyl ester against cisplatin-induced testis tissue damage in rats. Exp Ther Med. 15:3404–12. DOI:
10.3892/etm.2018.5819. PMID:
29545862. PMCID:
PMC5840930.



44. Yang WS, Jeong D, Yi YS, Park JG, Seo H, Moh SH, Hong S, Cho JY. 2013; IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediators Inflamm. 2013:518183. DOI:
10.1155/2013/518183. PMID:
24379523. PMCID:
PMC3863464.

45. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. 2016; Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016:7432797. DOI:
10.1155/2016/7432797. PMID:
27738491. PMCID:
PMC5055983.

46. Nemmar A, Al Hemeiri A, Al Hammadi N, Yuvaraju P, Beegam S, Yasin J, Elwasila M, Ali BH, Adeghate E. 2015; Early pulmonary events of nose-only water pipe (shisha) smoking exposure in mice. Physiol Rep. 3:e12258. DOI:
10.14814/phy2.12258. PMID:
25780090. PMCID:
PMC4393146.

47. Nemmar A, Yuvaraju P, Beegam S, John A, Raza H, Ali BH. 2013; Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am J Physiol Heart Circ Physiol. 305:H740–6. DOI:
10.1152/ajpheart.00200.2013. PMID:
23812392.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download