Abstract
Placental alterations are responsible for adverse pregnancy outcomes like preeclampsia and intrauterine growth restriction. And yet, placenta toxicology has not become a fully-fledged toxicology field. Because placenta is very often seen only as a barrier between the mother and the fetus, there is a lack and therefore a need for an experimental human model with technical recommendations to study placenta toxicology. In vitro approaches are recommended in experimental toxicology as they focus on a specific biological process and yield high-throughput screening methods. In the present study, we first established incubation conditions to preserve signatures of the human JEG-3 cell line identity while enabling toxicity detection. JEG-3 cells prepared in our incubation conditions were renamed JEG-Tox cells. As placental alterations are mainly triggered by uncontrolled apoptosis, we second used known apoptotic agents pregnant women are exposed to, to check that JEG-Tox cells can trigger apoptosis. Ethanol, bisphenol F, quinalphos, 4,4’-DDT, benzalkonium chloride, phenoxyethanol, propylparaben, and perfluorooctanic acid all induced chromatin condensation in JEG-Tox cells. Our incubation conditions allow JEG-Tox cells to keep placental cell identity and to respond to toxic chemicals. JEG-Tox cells are a pertinent model for placenta toxicology and could be used to better understand pregnancy alterations.
Placenta supports the normal growth and development of the fetus by coordinating gas exchanges, metabolic transfer, immunological functions and by producing, metabolizing and regulating numerous hormones including polypeptide and steroid hormones [1, 2]. Due to the key role of placenta during pregnancy, placental alterations are responsible for many adverse pregnancy outcomes like preeclampsia and intrauterine growth restriction [3-5]. The role of placenta goes beyond pregnancy since placental alterations can be risk factors for cognitive and visual development during childhood [6, 7]. And yet, placenta is more studied as a barrier between mother and fetus in the scientific literature than a target organ for toxic agents (Fig. 1).
Its major role in pregnancy disorders should encourage scientists to consider placenta as a critical organ to further study its response to toxic agents. Hundreds of potential toxic chemicals used in daily life (food, cosmetics, drugs…) and environmental pollutants can be responsible for pregnancy disorders. Placental alterations then represent a growing concern worldwide and placenta toxicology is about to become a fully-fledged toxicology field like cardiotoxicology, hepatotoxicity, neurotoxicity… Placental apoptosis is increased in spontaneous abortion in the first trimester, preeclampsia, post-term pregnancies, and intra-uterine growth restriction [8, 9]. Apoptosis is therefore a key endpoint in the assessment of potential risks for placental toxicity by chemicals.
Placental toxicity is in need of extensive investigation. Several models of placenta are available for toxicology studies. In vivo models are mainly mice, rats and rabbits, but placenta is the organ that shows the greatest diversity across mammalian species in its morphology and tissue organization, mechanisms of implantation and invasion, and endocrine regulation [10-12]. Animal models are consequently not fully suitable for the assessment of toxicological risk in the human placenta. Efforts are besides made to eliminate the use of animals in testing over the past 35 years in accordance to the 3R principle (Reduce, Refine, Replace). Perfused human placenta is the only model that respects the anatomical features of the human placenta [13], but the problem lies in the limited access to whole placenta for obvious technical and logistical reasons. Perfused human placentas as long as placenta-derived primary cultures are therefore not appropriate for high throughput screening. Moreover, it is almost impossible to control the maternal exposition to drugs, chemicals, cosmetics and pollutants that could influence the placental response to the toxic agent being studied. There are several different in vitro approaches currently available to evaluate chemicals toxicity in the human placenta. Immortalized cell lines can be bought in international cell banks like the American Type Culture Collection ATCC, the four main cell lines being HTR-8/SVneo, BeWo, JAR and JEG-3 trophoblastic cells. Contrary to BeWo, JAR and JEG-3 cell lines, the HTR-8/SVneo is not derived from a choriocarcinoma and was immortalized by SV-40 transfection. They show lower expression of proteins expressed by trophoblasts like cytokeratin-7 (CK7) and E-cadherin than BeWo, JEG-3 and JAR cell lines [14]. BeWo and JAR cells are less differentiated than JEG-3 cells. BeWo is best suited for studies on syncytial fusion as they are the only one to be fusigenic. JAR monolayers are unstable, as continued proliferation results in the formation of multilayers where ZO-1 and E-cadherin are lost from the cell surface [15]. With respect to TransEpithelial Electrical Resistance and electrical behaviour, JEG-3 are more similar to primary cells than BeWo and JAR cells [16]. For all these reasons, the human placental JEG-3 cell line is closer to human physiology than the other placenta cell lines and appears to be the best tool for the assessment of chemical toxicity in placenta. According to ATCC’s instructions, JEG-3 cells are grown in MEM culture medium supplemented with 10% fetal bovine serum (FBS) to bring a large number of nutritional and macromolecular factors essential for cell growth and promote cell proliferation. Fetal serum may bind or adsorb chemicals and then mask their cytotoxicity [17, 18]. Low serum medium should be preferred in toxicological studies but how low is the question. We previously published numerous studies using different cell lines (tenocytes, corneal and retinal epithelial cells, macrophages and keratinocytes) that revealed the toxicity of chemicals in culture medium supplemented with serum reduced from 10% to 2.5% [19-22]. Based on our expertise, we suggest that the concentration of serum in JEG-3 cells should be reduced to 2.5% for toxicology studies.
The objective of the present paper is to establish incubation conditions for JEG-3 placental cells to reveal pregnancy disorders induced by chemicals. To achieve this objective, we first studied JEG-3 cells behavior in 2.5% serum compared to 10%, and second, we checked that JEG-3 cells are able to undergo apoptosis in response to chemicals toxic for pregnant women.
All tested chemicals were purchased from Merck (Darmstadt, Germany) except ethanol (VWR Chemicals, Radnor, PA, USA) and perfluorooctanoic acid (PFOA; ThermoFisher Scientific, Waltham, MA, USA).
All cell culture reagents were obtained from Gibco (Paisley, UK). A 96-well microplates were purchased from Corning (Amsterdam, The Nederlands) and Nunc Lab-Tek II Chamber Slide system from Merck.
Antibodies were purchased from Merck (mouse anti-CK7 antibody) and ThermoFisher Scientific (Alexa Fluor 488 goat anti-mouse antibody and isotypic control). Fluorescent probes were obtained from ThermoFisher Scientific.
Fluorescence Resonance Energy Transfer (FRET) and ELISA kits were purchased from Cisbio Biosassays (Codolet, France) and MyBioSource (Vancouver, BC, Canada), respectively.
The choriocarcinoma-derived JEG-3 cell-line (ATCC HTB-36, Manassas, VA, USA), was grown as recommended by ATCC: Minimum Essential Medium Eagle’s medium supplemented with 10% FBS, 2 mM of glutamine, 50 IU/ml of penicillin and 50 IU/ml of streptomycin. Cells were detached using trypsin, counted, and then seeded at 80,000 cells/ml in 96-well microplates (200 μl by well) and Nunc Lab-Tek II Chamber Slide system for immunostaining.
Cells were cultured in three different concentrations of FBS (using the same batch): 0%, 2.5% and 10%. At 24 and 72 hours, cells were detached using trypsin and then counted by the Countess II Automated Cell Counter (ThermoFisher Scientific).
Cell line DNA was profiled by Short Tandem Repeat (STR). This technique also checks the lack of cellular cross-contamination [23]. STR analysis was performed by the Human STR Profiling Cell Authentication Service of ATCC.
The CK7 intermediate filament is an established marker of trophoblastic cells [14, 24]. A 24 hours after seeding in culture medium supplemented with 2.5 or 10% FBS, JEG-3 cells were fixed in 4% paraformaldehyde for 20 minutes, permeabilized in 0.1% Triton X-100 for 10 minutes, saturated with a solution of 1% BSA and 0.1% Tween in PBS for 2 hours, and then incubated overnight at 4°C with mouse anti-CK7 antibody (196 μg/ml) diluted in PBS containing 1% BSA and 0.1% Tween 20. After washing, the cells were incubated with Alexa Fluor 488 goat anti-mouse antibody (4 μg/ml) diluted in PBS containing 1% BSA for 2 hours at room temperature. Nuclei were stained with 300 nM DAPI for 5 minutes and Vectashield (Vector Laboratories, Burlingame, CA, USA) mounting medium was used for microscopy images (EVOS FL, ThermoFisher Scientific). Mouse IgG1 kappa clone P3.6.2.8.1 was used as an isotypic control to help differentiate non-specific background signal from specific antibody signal.
After 72 hours of incubation in cell culture medium supplemented with 2.5% or 10% FBS, microplates were centrifuged, and cell supernatants were collected. Estradiol was quantified in cell supernatants by FRET technology (HTRF Cisbio Biosassays, Codolet, France) according to manufacturer’s instructions. The detection limit of this assay is 20 pg/ml.
Human placental lactogen (hPl) hormone and human hyperglycosylated Chorionic Gonadotropin (hCG) hormone were measured by sandwich ELISA (MyBioSource) according to manufacturer’s instructions. Sensitivities are <46.875 pg/ml and 39 pg/ml for hPl and hCG dosage, respectively.
Cells were incubated with sodium lauryl sulfate or PFOA diluted in culture medium supplemented with either 2.5% or 10% FBS. After 24 hours, cell viability was evaluated using the neutral red assay. Neutral Red solution at 0.4% in water was diluted in culture medium with a ratio of 1:79 to give a final concentration of 50 μg/ml. Neutral Red was distributed in the plates for a 3-hour incubation time at 37°C. The cells were then rinsed with PBS to remove any remaining unincorporated dye. The dye was then released from the cells using a lysis solution (1% acetic acid, 50% ethanol and 49% H2O) and the fluorescence was measured (λex=540 nm, λem=600 nm) using Spark microplate fluorometer (Tecan, Männedorf, Switzerland).
Tested toxic agents for apoptosis evaluation were detailed in Table 1. Solvents were evaluated alone to discriminate their potential effect (data not shown).
Known apoptotic agents were diluted in culture medium supplemented with 2.5% FBS and incubated for 24 hours. Before running the apoptosis assay, cell viability was determined using the Alamar blue assay to eliminate necrotic concentrations and only keep subcytotoxic concentrations of the agents. Alamar blue was diluted in culture medium to a working concentration of 9 μg/ml. The cells were incubated with the solution for 6 hours at 37°C. The fluorescence signal was read (λex=535 nm, λem=600 nm) using the Spark cytofluorometer.
The UV fluorescent probe Hoechst 33342 enters living and apoptotic cells, intercalating into DNA. The fluorescent signal is proportional to chromatin condensation in apoptosis. The cells were incubated with Hoechst 33342 at 10 μg/ml for 30 minutes at room temperature. The fluorescence signal was read (λex=360 nm, λem=460 nm) using a cytofluorometer (Spark).
Means of at least three independent experiments were calculated and normalized to control. A one-way ANOVA followed by Dunnett’s test were performed (α risk=5%) using GraphPad Prism 6 software (San Diego, CA, USA). Thresholds of significance were *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 compared to control.
Three percentages of FBS were used: 0%, 2.5%, and 10% in culture medium (Fig. 2).
The percentage of living cells was dramatically decreased after 24 hours in culture medium without FBS (0% FBS); as expected, JEG-3 cells were not able to proliferate without FBS due to a lack of nutritional and macromolecular factors. JEG-3 cell proliferation in culture medium supplemented with 2.5% was similar to proliferation in 10% FBS at 24 and 72 hours.
The STR analysis was performed to compare nine STR core markers in JEG-3 cells in culture medium supplemented with 2.5% FBS to JEG-3 cells in 10% FBS (Table 2).
JEG-3 cells in 10% or 2.5% FBS expressed the same STR core markers. Reducing the percentage of FBS in culture medium of JEG-3 cells had no impact on DNA specific loci.
CK7 is a well-known epithelial marker for trophoblast cells and is known to be expressed in JEG-3 cells cultured in 10% FBS [14]. According to our microscopic observations, JEG-3 cells expressed similar levels of CK7 in 2.5% FBS and 10% FBS (Fig. 3A). The expression of CK7 was quantified using ImageJ software (Fig. 3B) and no statistical differences were observed between 2.5% FBS and 10% FBS.
We compared the secretion of placental hormones by JEG-3 cells in culture medium supplemented with 10% FBS to JEG-3 cells in 2.5% FBS. After 24 hours in either medium, the levels of each hormone were comparable (Table 3).
We compared sodium lauryl sulfate (SLS) and PFOA cytotoxicity in 2.5% FBS and 10% FBS, respectively (Fig. 4). At all tested concentrations, SLS diluted in culture medium supplemented with 10% FBS had no effect on JEG-3 cell viability. On the contrary, SLS diluted in culture medium supplemented with 2.5% FBS induced cytotoxicity at 30 μg/ml (37% of living cells, Fig. 4A) and 50 μg/ml (10% of living cells). PFOA cytotoxicity was observed at 80 μg/ml and 120 μg/ml in FBS 2.5% (68% and 27% of living cells, respectively, Fig. 4B) whereas only a slight loss of cell viability was observed at 120 μg/ml in FBS 10% (85% of viable cells). The classic concentration of FBS used for cell culture (10% of total volume) tends to mask SLS and PFOA cytotoxicity contrary to reduced FBS concentration (2.5%).
Based on our results, we pursue our study only using culture medium supplemented with FBS 2.5%; we renamed cells with these incubation conditions JEG-Tox.
We studied chromatin condensation in JEG-Tox cells after incubation with apoptotic chemicals. Before assessing chromatin condensation, we selected subcytotoxic concentrations i.e. concentrations that result in % of living cells higher than 70 (data not shown). This threshold is recommended in ISO standards and OECD guidelines that assess cytotoxicity on monolayer cells. Subcytotoxic concentrations ranged from 0.1% to 5% for ethanol, from 0.03 to 150 μg/ml for quinalphos, from 2 to 20 μg/ml for bisphenol F, from 0.4 to 16 μg/ml for 4,4’DDT, from 0.1 to 2.5 μg/ml for BAC, from 0.0001% to 0.15% for phenoxyethanol, from 0.2 to 20 μg/ml for propylparaben and from 0.04 to 100 μg/ml for PFOA.
As shown in Fig. 5, all the apoptotic chemicals significantly induced chromatin condensation in JEG-Tox cells. Chromatin condensation was initiated with ethanol 2.5%, quinalphos 0.3 μg/ml, bisphenol F 5 μg/ml, 4,4’DDT 2 μg/ml, BAC 2.5 μg/ml, phenoxyethanol 0.15%, propylparaben 20 μg/ml and PFOA 20 μg/ml; all those concentrations being in accordance with the literature in other cell types [25-31].
Chemicals are more concentrated in the placenta than in maternal tissues [32]. Exposure of pregnant women to hazardous chemicals and environmental pollutants like alcohol, pesticides, preservatives, or plasticizers can lead to decreased birth length and weight and increased infant mortality, alterations of developing nervous system and other vital organs, endocrine disruptions [33-36].
Proteins present in FBS can bind chemicals thus masking their potential cytotoxicity and affecting cell response. It was previously proposed that the protein corona formed around particles greatly influences particle toxicity [37]. High FBS concentrations used in growth medium (mainly 10%) are therefore not suitable for toxicity studies. Some of our previous studies on ocular and skin cell lines demonstrated that 2.5% FBS is a good compromise as serum total deprivation induces cell death [38-40]. In this study, we compared placental JEG-3 cells behaviour in 2.5% FBS versus 10% FBS. We first evaluated cell proliferation and observed that JEG-3 cells cultured in 2.5% or 10% FBS have similar proliferation rates, and as expected, cells in 0% FBS did not survive. We second analysed STR core markers and concluded that JEG-3 cells had the same STR core markers and thus the same genotype whether they are cultured in 2.5% or in 10% FBS. We third performed immunochemistry studies to ensure that JEG-3 cells in 2.5% FBS express CK7, a known marker of placental cells. Our results showed that reducing the percentage of FBS in JEG-3 cells does not alter signatures of cell identity such as cell proliferation rate, DNA profile and specific protein expression. JEG-3 cells in 2.5% FBS released similar levels of hCG, hPL, and estradiol to JEG-3 cells in 10% FBS, and thus maintain the endocrine function of human placenta.
In the cytotoxicity study, we didn’t observe any cell death when SLS was diluted in 10% FBS up to 50 μg/ml whereas when it was diluted in 2.5% FBS, SLS induced a dramatic loss of cell viability at 30 μg/ml. Cytotoxicity of PFOA was revealed at 200 μM when it was diluted in 2.5% FBS whereas only a slight loss of cell viability was observed at 300 μM when it was diluted in 10% FBS. It appears that JEG-3 cells in 2.5% FBS are more suitable for toxicological studies than JEG-3 cells in 10% FBS. We renamed JEG-3 cells in 2.5% FBS JEG-Tox cells.
Apoptosis is suggested to be a key mechanism in placental dysfunction. A growing amount of data indeed suggests that uncontrolled placental apoptosis has side effects on both the placenta and maternal physiology [41]. To validate JEG-Tox cells as a pertinent model for the evaluation of placental toxicity, we checked whether they were able to trigger apoptosis after incubation with known apoptotic agents. We selected chemicals that pregnant women can be exposed to such as ethanol through alcohol consumption, preservatives present in cosmetics or drugs, pesticides and cookware coatings. In our experimental conditions, all the tested apoptotic chemicals induced chromatin condensation in JEG-Tox cells.
To conclude, reducing the percentage of FBS from 10%, which is the recommended concentration for cell growth, to 2.5% does not affect neither DNA profile, nor placental marker, nor hormone secretion, but reveals placental toxicity increasing cell sensitivity to chemicals contrary to FBS 10%. JEG-Tox cells can be of great value in placental toxicological studies, especially to study apoptosis that is at the origin of numerous severe pregnancy disorders.
Acknowledgements
We acknowledge support from Adebiopharm ER67 (Paris) and Troy Green for his linguistic support.
Notes
References
1. Griffiths SK, Campbell JP. 2015; Placental structure, function and drug transfer. Contin Educ Anaesth Crit Care Pain. 15:84–9. DOI: 10.1093/bjaceaccp/mku013.

2. Murphy VE, Smith R, Giles WB, Clifton VL. 2006; Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 27:141–69. DOI: 10.1210/er.2005-0011. PMID: 16434511.


3. Chaddha V, Viero S, Huppertz B, Kingdom J. 2004; Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 9:357–69. DOI: 10.1016/j.siny.2004.03.006. PMID: 15691771.


4. Fisher SJ. 2004; The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2:53. DOI: 10.1186/1477-7827-2-53. PMID: 15236649. PMCID: PMC493282.


5. Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G. 2016; Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 215(1 Suppl):S1–46. DOI: 10.1016/j.ajog.2016.03.001. PMID: 26972897. PMCID: PMC4925329.

6. Innis SM. 2007; Fatty acids and early human development. Early Hum Dev. 83:761–6. DOI: 10.1016/j.earlhumdev.2007.09.004. PMID: 17920214.


7. Zhu Y, Mordaunt CE, Yasui DH, Marathe R, Coulson RL, Dunaway KW, Jianu JM, Walker CK, Ozonoff S, Hertz-Picciotto I, Schmidt RJ, LaSalle JM. 2019; Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Hum Mol Genet. 28:2659–74. DOI: 10.1093/hmg/ddz084. PMID: 31009952. PMCID: PMC6687952.



8. Smith SC, Baker PN, Symonds EM. 1997; Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 177:1395–401. DOI: 10.1016/S0002-9378(97)70081-4. PMID: 9423741.


9. Erel CT, Dane B, Calay Z, Kaleli S, Aydinli K. 2001; Apoptosis in the placenta of pregnancies complicated with IUGR. Int J Gynaecol Obstet. 73:229–35. DOI: 10.1016/S0020-7292(01)00373-3. PMID: 11376669.


10. Enders AC, Blankenship TN. 1999; Comparative placental structure. Adv Drug Deliv Rev. 38:3–15. DOI: 10.1016/S0169-409X(99)00003-4. PMID: 10837743.


11. Malassiné A, Frendo JL, Evain-Brion D. 2003; A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 9:531–9. DOI: 10.1093/humupd/dmg043. PMID: 14714590.

12. Schmidt A, Morales-Prieto DM, Pastuschek J, Fröhlich K, Markert UR. 2015; Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol. 108:65–71. DOI: 10.1016/j.jri.2015.03.001. PMID: 25817465.


13. Ceccaldi PF, Mandelbrot L, Farinotti R, Forestier F, Gil S. 2010; [Contributions of the ex vivo human perfused placenta in the study of placental transfer of drugs]. J Gynecol Obstet Biol Reprod (Paris). 39:601–5. French. DOI: 10.1016/j.jgyn.2010.06.010. PMID: 20692775.

14. Abou-Kheir W, Barrak J, Hadadeh O, Daoud G. 2017; HTR-8/SVneo cell line contains a mixed population of cells. Placenta. 50:1–7. DOI: 10.1016/j.placenta.2016.12.007. PMID: 28161053.


15. Mitchell AM, Yap AS, Payne EJ, Manley SW, Mortimer RH. 1995; Characterization of cell polarity and epithelial junctions in the choriocarcinoma cell line, JAR. Placenta. 16:31–9. DOI: 10.1016/0143-4004(95)90079-9. PMID: 7716126.


16. Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B, Ertl P. 2017; A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci Rep. 7:5892. DOI: 10.1038/s41598-017-06364-z. PMID: 28724925. PMCID: PMC5517571.



17. Bohets HH, Nouwen EJ, De Broe ME, Dierickx PJ. 1994; Effects of foetal calf serum on cell viability, cytotoxicity and detoxification in the two kidney-derived cell lines LLC-PK1 and MDCK. Toxicol In Vitro. 8:559–61. DOI: 10.1016/0887-2333(94)90016-7. PMID: 20692960.


18. Lordan S, Higginbotham CL. 2012; Effect of serum concentration on the cytotoxicity of clay particles. Cell Biol Int. 36:57–61. DOI: 10.1042/CBI20100587. PMID: 21883092.


19. Pouzaud F, Dutot M, Martin C, Debray M, Warnet JM, Rat P. 2006; Age-dependent effects on redox status, oxidative stress, mitochondrial activity and toxicity induced by fluoroquinolones on primary cultures of rabbit tendon cells. Comp Biochem Physiol C Toxicol Pharmacol. 143:232–41. DOI: 10.1016/j.cbpc.2006.02.006. PMID: 16574493.


20. Dutot M, Fagon R, Hemon M, Rat P. 2012; Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from brown seaweed Ascophyllum nodosum. Appl Biochem Biotechnol. 167:2234–40. DOI: 10.1007/s12010-012-9761-1. PMID: 22692848.


21. Wakx A, Dutot M, Massicot F, Mascarelli F, Limb GA, Rat P. 2016; Amyloid β peptide induces apoptosis through P2X7 cell death receptor in retinal cells: modulation by marine omega-3 fatty acid DHA and EPA. Appl Biochem Biotechnol. 178:368–81. DOI: 10.1007/s12010-015-1878-6. PMID: 26467741. PMCID: PMC4718936.


22. Ghazi K, Deng-Pichon U, Warnet JM, Rat P. 2012; Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS One. 7:e48351. DOI: 10.1371/journal.pone.0048351. PMID: 23173033. PMCID: PMC3500239.
23. Reid Y, Storts D, Riss T, Minor L. c2004. Authentication of human cell lines by STR DNA profiling analysis [Internet]. Eli Lilly & Company and the National Center for Advancing Translational Sciences;Bethesda, MD: Available from: http://www.ncbi.nlm.nih.gov/books/NBK144066/. cited 2019 Jul 19.
24. Maldonado-Estrada J, Menu E, Roques P, Barré-Sinoussi F, Chaouat G. 2004; Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J Immunol Methods. 286:21–34. DOI: 10.1016/j.jim.2003.03.001. PMID: 15087219.

25. Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszeń M. 2008; Zinc inhibits ethanol-induced HepG2 cell apoptosis. Toxicol Appl Pharmacol. 229:1–9. DOI: 10.1016/j.taap.2007.11.019. PMID: 18396304.


26. Zerin T, Song HY, Kim YS. 2015; Quinalphos induced intracellular ROS generation and apoptosis in human alveolar A549 cells. Mol Cell Toxicol. 11:61–9. DOI: 10.1007/s13273-015-0008-4.

27. Mokra K, Kocia M, Michałowicz J. 2015; Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study). Food Chem Toxicol. 84:79–88. DOI: 10.1016/j.fct.2015.08.007. PMID: 26271707.

28. Carvalho CM, Menezes PF, Letenski GC, Praes CE, Feferman IH, Lorencini M. 2012; In vitro induction of apoptosis, necrosis and genotoxicity by cosmetic preservatives: application of flow cytometry as a complementary analysis by NRU. Int J Cosmet Sci. 34:176–82. DOI: 10.1111/j.1468-2494.2011.00698.x. PMID: 22118339.

29. Eggert A, Cisneros-Montalvo S, Anandan S, Musilli S, Stukenborg JB, Adamsson A, Nurmio M, Toppari J. 2019; The effects of perfluorooctanoic acid (PFOA) on fetal and adult rat testis. Reprod Toxicol. 90:68–76. DOI: 10.1016/j.reprotox.2019.08.005. PMID: 31412280.


30. Jin X, Song L, Liu X, Chen M, Li Z, Cheng L, Ren H. 2014; Protective efficacy of vitamins C and E on p,p'-DDT-induced cytotoxicity via the ROS-mediated mitochondrial pathway and NF-κB/FasL pathway. PLoS One. 9:e113257. DOI: 10.1371/journal.pone.0113257. PMID: 25464339. PMCID: PMC4252254.

31. Pozarowska D, Pozarowski P. 2011; Benzalkonium chloride (BAK) induces apoptosis or necrosis, but has no major influence on the cell cycle of Jurkat cells. Folia Histochem Cytobiol. 49:225–30. DOI: 10.5603/FHC.2011.0031. PMID: 21744321.


32. Gupta RK, Gupta RC. Gupta RC, editor. 2017. Placental toxicity. Reproductive and Developmental Toxicology. 2nd ed. Academic Press;London: p. 1301–25. DOI: 10.1016/B978-0-12-804239-7.00068-8.

33. Nykjaer C, Alwan NA, Greenwood DC, Simpson NA, Hay AW, White KL, Cade JE. 2014; Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J Epidemiol Community Health. 68:542–9. DOI: 10.1136/jech-2013-202934. PMID: 24616351. PMCID: PMC4033207.



34. Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, Vélez Vega C, Cordero JF, Alshawabkeh A, Meeker JD. 2019; Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int. 132:105099. DOI: 10.1016/j.envint.2019.105099. PMID: 31430608. PMCID: PMC6754790.

35. Ferguson KK, McElrath TF, Meeker JD. 2014; Environmental phthalate exposure and preterm birth. JAMA Pediatr. 168:61–7. DOI: 10.1001/jamapediatrics.2013.3699. PMID: 24247736. PMCID: PMC4005250.



36. Barkoski JM, Busgang SA, Bixby M, Bennett D, Schmidt RJ, Barr DB, Panuwet P, Gennings C, Hertz-Picciotto I. 2019; Prenatal phenol and paraben exposures in relation to child neurodevelopment including autism spectrum disorders in the MARBLES study. Environ Res. 179(Pt A):108719. DOI: 10.1016/j.envres.2019.108719. PMID: 31627027. PMCID: PMC6948181.

37. Herzog E, Byrne HJ, Davoren M, Casey A, Duschl A, Oostingh GJ. 2009; Dispersion medium modulates oxidative stress response of human lung epithelial cells upon exposure to carbon nanomaterial samples. Toxicol Appl Pharmacol. 236:276–81. DOI: 10.1016/j.taap.2009.02.007. PMID: 19233222.


38. Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N. 2005; Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 46:1330–8. DOI: 10.1167/iovs.04-0363. PMID: 15790899.


39. Koyama Y, Kimura Y, Yoshioka Y, Wakamatsu D, Kozaki R, Hashimoto H, Matsuda T, Baba A. 2000; Serum-deprivation induces cell death of rat cultured microglia accompanied with expression of Bax protein. Jpn J Pharmacol. 83:351–4. DOI: 10.1016/S0021-5198(19)30572-4. PMID: 11001183.


40. Kulkarni GV, McCulloch CA. 1994; Serum deprivation induces apoptotic cell death in a subset of Balb/c 3T3 fibroblasts. J Cell Sci. 107(Pt 5):1169–79. PMID: 7929626.
41. Sharp AN, Heazell AE, Crocker IP, Mor G. 2010; Placental apoptosis in health and disease. Am J Reprod Immunol. 64:159–69. DOI: 10.1111/j.1600-0897.2010.00837.x. PMID: 20367628. PMCID: PMC3025811.



Fig. 1
Comparison of Pubmed publications using “placenta barrier” or “placenta target organ” as keywords in the research tool.
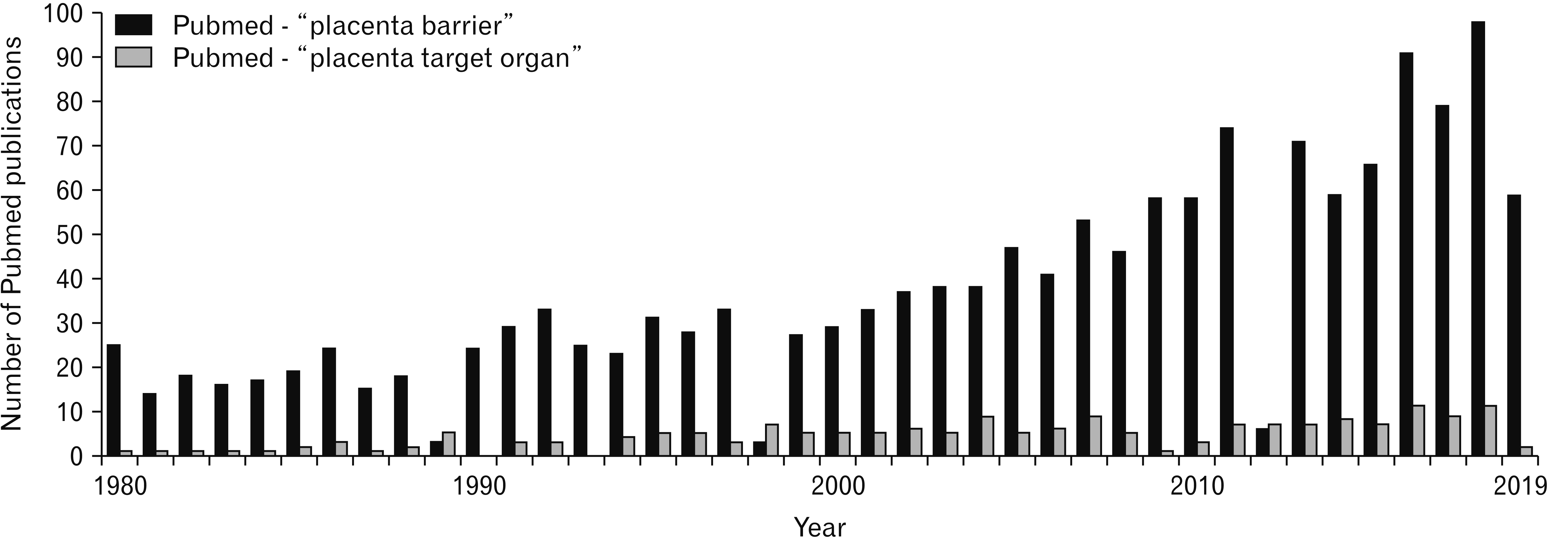
Fig. 2
Proliferation of JEG-3 cells in culture medium supplemented with different FBS concentrations. JEG-3 cells were incubated with three different concentrations of FBS for 24 or 72 hours, cell count was conducted to quantify the effect of FBS on JEG-3 cell proliferation. Black: 10% FBS, grey: 2.5% FBS, light grey: 0% FBS. FBS, fetal bovine serum; NS, not significant.
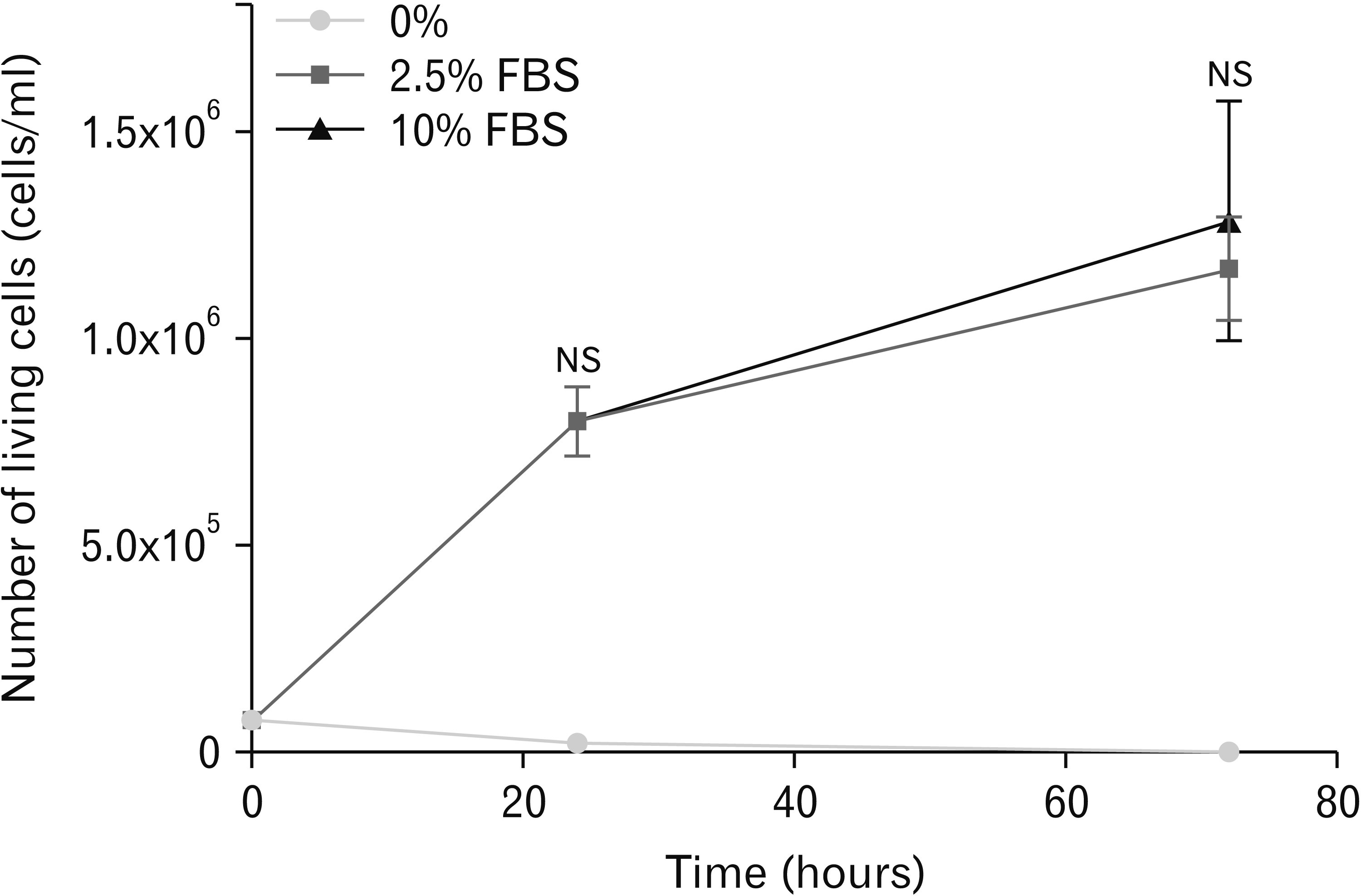
Fig. 3
Expression of CK7 in JEG-3 cells in 2.5% and 10% FBS. (A) Microscopic observation of JEG-3 cells stained with either anti-CK7 antibody or isotype control and then stained with Alexa Fluor 488 (green). DAPI was used to stain DNA (blue) (Magnification, ×200). Data are representative of at least 3 independent experiments. (B) Quantification of CK7 fluorescence using ImageJ software. Normalized CK7 fluorescence intensity was obtained by dividing green fluorescence intensity by blue fluorescence intensity to take into account the difference in cell numbers in the selected microscopic fields. CK7, cytokeratin-7; FBS, fetal bovine serum; NS, not significant. Scale bar=100 μm (A).
Fig. 4
Comparison of JEG-3 cell viability after incubation with SLS (A) or PFOA (B) in FBS 10% or FBS 2.5%. JEG-3 cells were incubated with SLS from 10 to 50 μg/ml or PFOA from 40 to 120 μM for 24 hours. Cell viability was determined using the neutral red assay. ££££P<0.0001 compared to negative control in 10% FBS, ****P<0.0001 compared to negative control in 2.5% FBS (n=3). FBS, fetal bovine serum; PFOA, perfluorooctanic acid; SLS, sodium lauryl sulfate.
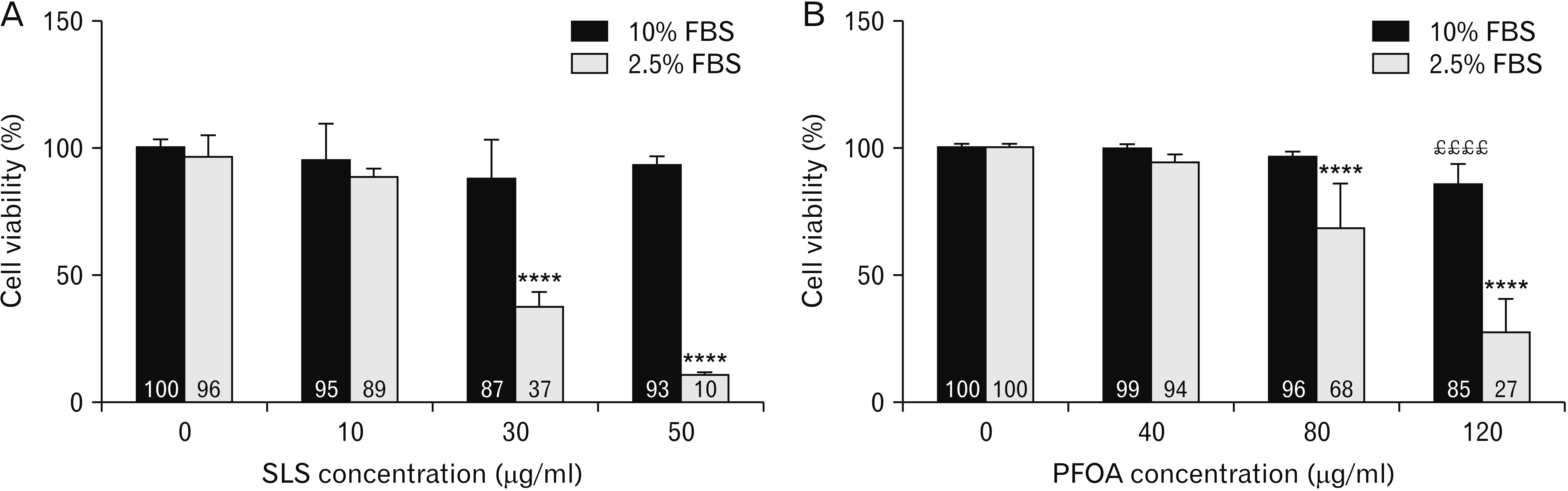
Fig. 5
Evaluation of cell viability and chromatin condensation of JEG-Tox cells after incubation with apoptosis inducers for 24 hours. Cell viability and chromatin condensation were quantified using the Alamar blue and Hoechst 33342 assays, respectively. Dashed line: cell viability, solid line: chromatin condensation. *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 compared to negative control (n=3). BAC, benzalkonium chloride; PFOA, perfluorooctanic acid; 4,4’DDT, 4,4’-dichlorodiphenyltrichloroethane.
Table 1
Tested toxic agents for apoptosis evaluation
Table 2
STR analysis of JEG-3 cells cultured in culture medium supplemented with either 10% or 2.5% FBS




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download