Introduction
Materials and Methods
Design and group allocation
Recruitment and ethics
Inclusion and exclusion criteria
Extraction of the spine and cancellous bone
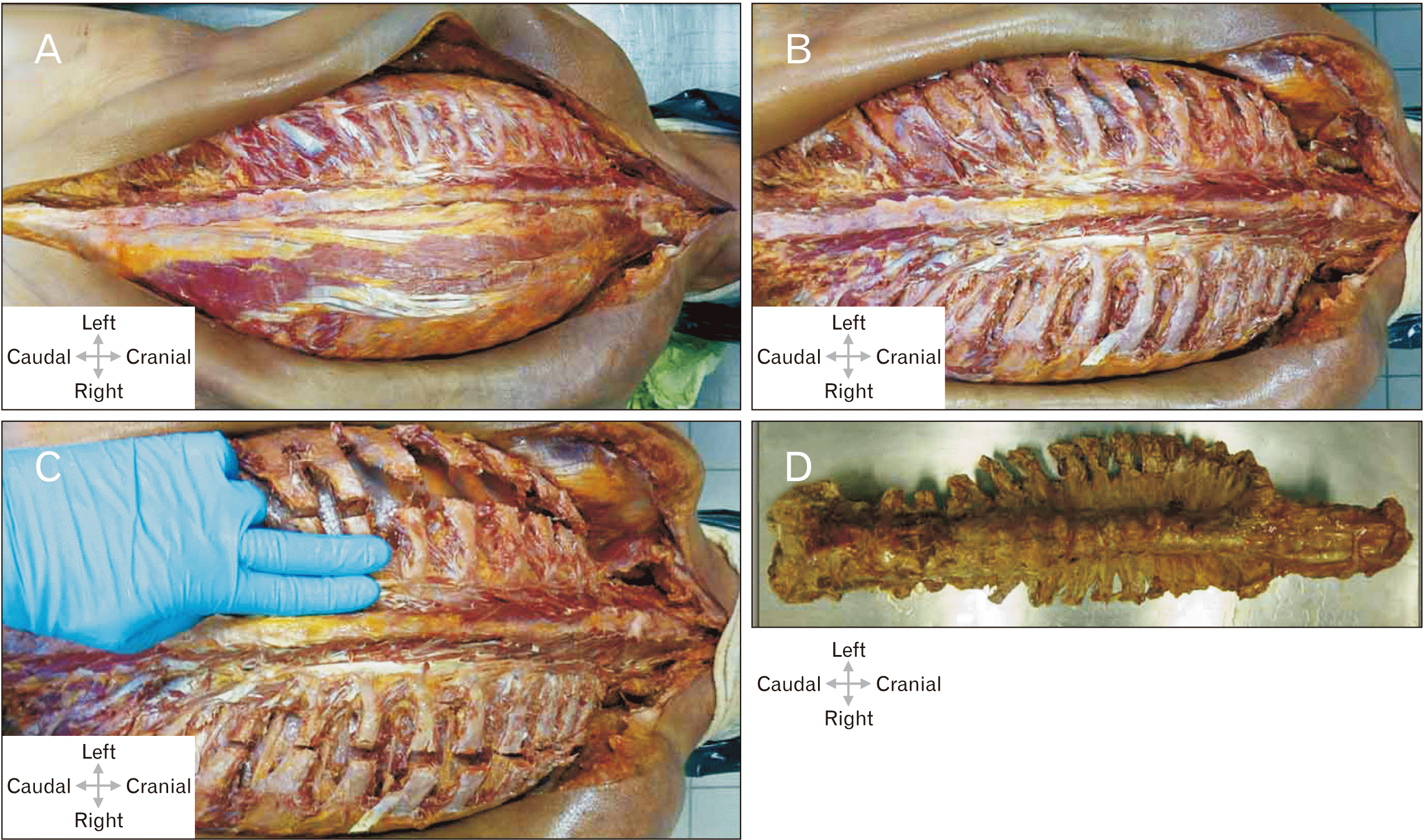 | Fig. 1(A) Skin incision along the spinous process from the sacrum to the occiput (superior nuchal line); secondary spinal muscles (including the trapezius muscle, the latissimus dorsi, major and minor rhomboid, superior and inferior posterior serratus muscles), skin and subcutaneous fatty tissue were shifted laterally. Left: the subsequent lateral tract of the autochthonous spinal muscles was removed and the ribs were exposed. (B) The spinal muscles have been largely removed; the intercostal spaces have been emptied in the paravertebral aspect. The pleura has been separated from the inside of the ribs, the atlanto-occipital joint is exposed and the capsular ligaments have been transected. The cervical spine has been mobilized in the retropharyngeal space, the scalenemuscles have been transected. (C) Transection of all ribs about 1.5 inches wide in the paravertebral aspect of the spinous processes, along the scapular line. Separation of the parietal pleura from the inside of the ribs, wedge-shaped incision in the sacrum. (D) Complete specimen of the spine. |
Diagnostic imaging
Computed tomography and quantitative computed tomography
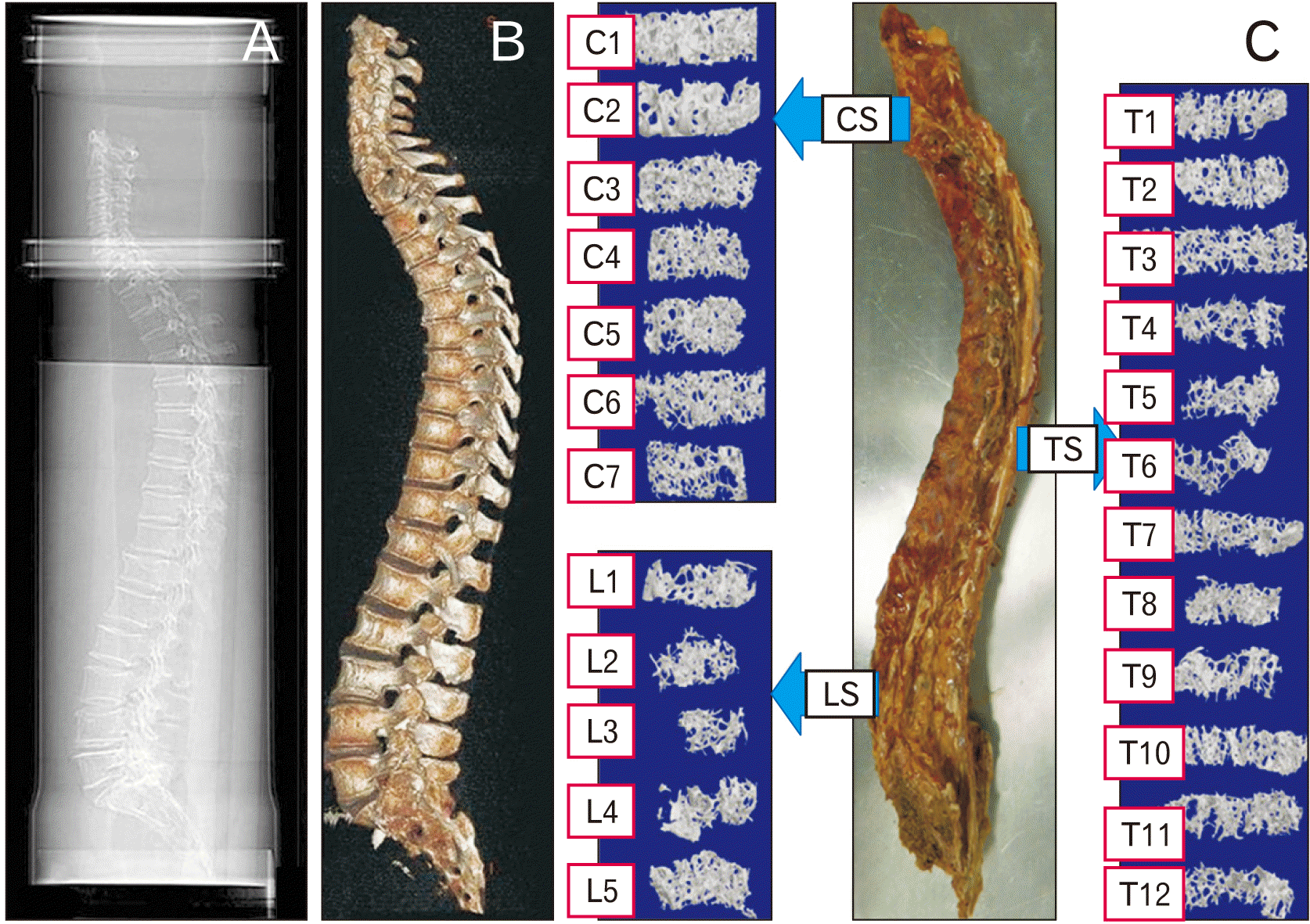 | Fig. 2(A) Spine in a water bath, registration of vertebral deformities on the lateral image. (B) Three-dimensional reconstruction to visualize the overall anatomy of the spine. (C) Prepared spinal column with reconstructed cancellous bone cylinders in micro-computed tomography. CS, cervical spine; LS, lumbar spine; TS, thoracic spine. |
Micro-computed tomography images and evaluation of microarchitecture
Statistics
Results
Table 1
| Medical history | Overall group (n=10) |
|---|---|
| Age (yr) | 81.3±7.9 |
| Sex (male/female) | 4/6 |
| Body mass index (kg/m2) | 20.8±4.6 |
| Bone mineral density (mg/cm3)* | 54.5±20.0 |
| Segments excluded | C1–L5 |
| Vertebral body fractures (n) | 14 |
| Total number of vertebrae | 240 |
Quantitative computed tomography
Micro-computed tomography
Group comparison of the spinal sections
 | Fig. 3(A) Regional variation in vertebral trabecular BVF. Cervical vertebrae exhibit significantly higher BVF in relation to thoracic and lumbar vertebrae (Kruskal-Wallis-test, P-values in Table 2). The subgroup analysis using the Mann-Whitney U-test showed no significant difference between women and men or between those over and under 80 years of age (P>0.05). (B) Regional variation in Tb.Th. Cervical vertebrae exhibit significantly higher Tb.Th in relation to thoracic and lumbar vertebrae. Only in the over-80s is there no significant difference between the cervical and lumbar vertebrae (Kruskal-Wallis-test, P-values in Table 2). The subgroup analysis using the Mann-Whitney U-test showed no significant difference between women and men or between those over and under 80 years of age (P>0.05). (C) Regional variation in DA. Cervical vertebrae exhibit significantly lower DA in relation to thoracic and lumbar vertebrae (ANOVA, post-hoc LSD test, P-values in Table 2). The subgroup analysis using the independent t-test showed a significant difference between women and men (P=0.042) and no significant difference between those over and under 80 years of age (P>0.05). (D) Regional variation in Tb.Sp. Cervical vertebrae exhibit significantly lower Tb.Sp in relation to thoracic and lumbar vertebrae (ANOVA, post-hoc LSD test, P-values in Table 2). The subgroup analysis using the independent t-test showed no significant difference between women and men or between those over and under 80 years of age (P>0.05). (E) Regional variation in Tb.N. Cervical vertebrae exhibit significantly higher Tb.N in relation to thoracic and lumbar vertebrae (ANOVA, post-hoc LSD test, P-values in Table 2). The subgroup analysis using the independent t-test showed no significant difference between women and men or between those over and under 80 years of age (P>0.05). BVF, bone volume fraction; DA, degree of anisotropy; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular bone thickness. Table 2Descriptive statistics for micro-computed tomography parameters
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subgroup analysis
Discussion
Discussion of results
Discussion of the method
Conclusions
BVF decreases from the cervical spine to the lumbar spine.
Trabecular thickness and trabecular separation are different in the individual portions of the spine. The differences are especially pronounced between the cervical and thoracic spine and the cervical and lumbar spine.
The degree of anisotropy is lower in the cervical spine than in the thoracic or lumbar spine.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download