1. Myer GD, Ford KR, Barber Foss KD, Goodman A, Ceasar A, Rauh MJ, Divine JG, Hewett TE. 2010; The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech (Bristol, Avon). 25:700–7. DOI:
10.1016/j.clinbiomech.2010.04.001. PMID:
20466469. PMCID:
PMC2900391.



2. Engelina S, Antonios T, Robertson CJ, Killingback A, Adds PJ. 2014; Ultrasound investigation of vastus medialis oblique muscle architecture: an
in vivo study. Clin Anat. 27:1076–84. DOI:
10.1002/ca.22413. PMID:
24797580.

3. Arazpour M, Bahramian F, Abutorabi A, Nourbakhsh ST, Alidousti A, Aslani H. 2016; The effect of patellofemoral pain syndrome on gait parameters: a literature review. Arch Bone Jt Surg. 4:298–306. PMID:
27847840. PMCID:
PMC5100443.


4. Arazpour M, Notarki TT, Salimi A, Bani MA, Nabavi H, Hutchins SW. 2013; The effect of patellofemoral bracing on walking in individuals with patellofemoral pain syndrome. Prosthet Orthot Int. 37:465–70. DOI:
10.1177/0309364613476535. PMID:
23436695.


5. Johnston LB, Gross MT. 2004; Effects of foot orthoses on quality of life for individuals with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 34:440–8. DOI:
10.2519/jospt.2004.34.8.440. PMID:
15373007.


6. Dixit S, DiFiori JP, Burton M, Mines B. 2007; Management of patellofemoral pain syndrome. Am Fam Physician. 75:194–202. PMID:
17263214.

7. Murphy AC, Muldoon SF, Baker D, Lastowka A, Bennett B, Yang M, Bassett DS. 2018; Structure, function, and control of the human musculoskeletal network. PLoS Biol. 16:e2002811. DOI:
10.1371/journal.pbio.2002811. PMID:
29346370. PMCID:
PMC5773011.

8. Hortobágyi T, Garry J, Holbert D, Devita P. 2004; Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 51:562–9. DOI:
10.1002/art.20545. PMID:
15334428.

9. Lefebvre R, Leroux A, Poumarat G, Galtier B, Guillot M, Vanneuville G, Boucher JP. 2006; Vastus medialis: anatomical and functional considerations and implications based upon human and cadaveric studies. J Manipulative Physiol Ther. 29:139–44. DOI:
10.1016/j.jmpt.2005.12.006. PMID:
16461173.


10. Sattler M, Dannhauer T, Ring-Dimitriou S, Sänger AM, Wirth W, Hudelmaier M, Eckstein F. 2014; Relative distribution of quadriceps head anatomical cross-sectional areas and volumes--sensitivity to pain and to training intervention. Ann Anat. 196:464–70. DOI:
10.1016/j.aanat.2014.07.005. PMID:
25153247. PMCID:
PMC4250421.



11. Castanov V, Hassan SA, Shakeri S, Vienneau M, Zabjek K, Richardson D, McKee NH, Agur AMR. 2019; Muscle architecture of vastus medialis obliquus and longus and its functional implications: a three-dimensional investigation. Clin Anat. 32:515–23. DOI:
10.1002/ca.23344. PMID:
30701597.


12. Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sänger AM, Kwoh CK, Hunter DJ, Eckstein F. 2012; Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain- data from the osteoarthritis initiative. Osteoarthritis Cartilage. 20:532–40. DOI:
10.1016/j.joca.2012.02.635. PMID:
22395037. PMCID:
PMC3350840.


13. Glass NA, Torner JC, Frey Law LA, Wang K, Yang T, Nevitt MC, Felson DT, Lewis CE, Segal NA. 2013; The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: a 5-year longitudinal study. Osteoarthritis Cartilage. 21:1154–9. DOI:
10.1016/j.joca.2013.05.016. PMID:
23973125. PMCID:
PMC3774035.



14. Villafañe JH, Bissolotti L, La Touche R, Pedersini P, Negrini S. 2019; Effect of muscle strengthening on perceived pain and static knee angles in young subjects with patellofemoral pain syndrome. J Exerc Rehabil. 15:454–9. DOI:
10.12965/jer.1938224.112. PMID:
31316941. PMCID:
PMC6614779.



15. Abe T, Loenneke JP, Thiebaud RS. 2015; Morphological and functional relationships with ultrasound measured muscle thickness of the lower extremity: a brief review. Ultrasound. 23:166–73. DOI:
10.1177/1742271X15587599. PMID:
27433253. PMCID:
PMC4760590.



16. Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, Annoni R, Puthucheary Z, Gordon IR, Morris PE, Denehy L. 2015; Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 30:1151.e9–14. DOI:
10.1016/j.jcrc.2015.05.024. PMID:
26211979.

17. Grob K, Manestar M, Filgueira L, Ackland T, Gilbey H, Kuster MS. 2016; New insight in the architecture of the quadriceps tendon. J Exp Orthop. 3:32. DOI:
10.1186/s40634-016-0068-y. PMID:
27813020. PMCID:
PMC5095096.



18. Ayala-Mejias JD, Garcia-Gonzalez B, Alcocer-Perez-España L, Villafañe JH, Berjano P. 2017; Relationship between widening and position of the tunnels and clinical results of anterior cruciate ligament reconstruction to knee osteoarthritis: 30 patients at a minimum follow-up of 10 years. J Knee Surg. 30:501–8. DOI:
10.1055/s-0036-1593367. PMID:
27685765.


19. Ema R, Wakahara T, Hirayama K, Kawakami Y. 2017; Effect of knee alignment on the quadriceps femoris muscularity: cross-sectional comparison of trained versus untrained individuals in both sexes. PLoS One. 12:e0183148. DOI:
10.1371/journal.pone.0183148. PMID:
28806771. PMCID:
PMC5555710.

20. Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. 2017; ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 18:529. DOI:
10.1186/s12859-017-1934-z. PMID:
29187165. PMCID:
PMC5708080.



21. Franchi MV, Longo S, Mallinson J, Quinlan JI, Taylor T, Greenhaff PL, Narici MV. 2018; Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sports. 28:846–53. DOI:
10.1111/sms.12961. PMID:
28805932. PMCID:
PMC5873262.


22. Winby CR, Lloyd DG, Besier TF, Kirk TB. 2009; Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 42:2294–300. DOI:
10.1016/j.jbiomech.2009.06.019. PMID:
19647257.


23. Andriacchi TP, Koo S, Scanlan SF. 2009; Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 91(Suppl 1):95–101. DOI:
10.2106/JBJS.H.01408. PMID:
19182033. PMCID:
PMC2663350.


24. Englund M. 2010; The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 24:39–46. DOI:
10.1016/j.berh.2009.08.008. PMID:
20129198.


25. Kim D, Park G, Kuo LT, Park W. 2018; The effects of pain on quadriceps strength, joint proprioception and dynamic balance among women aged 65 to 75 years with knee osteoarthritis. BMC Geriatr. 18:245. DOI:
10.1186/s12877-018-0932-y. PMID:
30332992. PMCID:
PMC6192068.



26. Chopp-Hurley JN, Langenderfer JE, Dickerson CR. 2014; Probabilistic evaluation of predicted force sensitivity to muscle attachment and glenohumeral stability uncertainty. Ann Biomed Eng. 42:1867–79. DOI:
10.1007/s10439-014-1035-3. PMID:
24866570.


27. Grob K, Manestar M, Filgueira L, Kuster MS, Gilbey H, Ackland T. 2018; The interaction between the vastus medialis and vastus intermedius and its influence on the extensor apparatus of the knee joint. Knee Surg Sports Traumatol Arthrosc. 26:727–38. DOI:
10.1007/s00167-016-4396-3. PMID:
28124107.


28. Belli G, Vitali L, Botteghi M, Vittori LN, Petracci E, Latessa PM. 2015; Electromyographic analysis of leg extension exercise during different ankle and knee positions. J Mech Med Biol. 15:1540037. DOI:
10.1142/S0219519415400370.

29. Kang JI, Park JS, Choi H, Jeong DK, Kwon HM, Moon YJ. 2017; A study on muscle activity and ratio of the knee extensor depending on the types of squat exercise. J Phys Ther Sci. 29:43–7. DOI:
10.1589/jpts.29.43. PMID:
28210036. PMCID:
PMC5300802.



30. Muhamed R, Saralaya VV, Murlimanju BV, Chettiar GK. 2017; In vivo magnetic resonance imaging morphometry of the patella bone in South Indian population. Anat Cell Biol. 50:99–103. DOI:
10.5115/acb.2017.50.2.99. PMID:
28713612. PMCID:
PMC5509906.



31. Page BJ, Mrowczynski OD, Payne RA, Tilden SE, Lopez H, Rizk E, Harbaugh K. 2019; The relative location of the major femoral nerve motor branches in the thigh. Cureus. 11:e3882. DOI:
10.7759/cureus.3882. PMID:
30899633. PMCID:
PMC6420329.

32. Chaitow L, DeLany J. 2011. Clinical application of neuromuscular techniques volume 2, the lower body. 2nd ed. Churchill Livingstone;Edinburgh:
33. Toumi H, Poumarat G, Benjamin M, Best TM, F'Guyer S, Fairclough J. 2007; New insights into the function of the vastus medialis with clinical implications. Med Sci Sports Exerc. 39:1153–9. DOI:
10.1249/01.mss.0b013e31804ec08d. PMID:
17596784.


34. Chang WD, Huang WS, Lai PT. 2015; Muscle activation of vastus medialis oblique and vastus lateralis in sling-based exercises in patients with patellofemoral pain syndrome: a cross-over study. Evid Based Complement Alternat Med. 2015:740315. DOI:
10.1155/2015/740315. PMID:
26504480. PMCID:
PMC4609425.

35. Thomaes T, Thomis M, Onkelinx S, Coudyzer W, Cornelissen V, Vanhees L. 2012; Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD-patients. BMC Med Imaging. 12:7. DOI:
10.1186/1471-2342-12-7. PMID:
22471726. PMCID:
PMC3342139.



36. Stevens DE, Smith CB, Harwood B, Rice CL. 2014; In vivo measurement of fascicle length and pennation of the human anconeus muscle at several elbow joint angles. J Anat. 225:502–9. DOI:
10.1111/joa.12233. PMID:
25223934. PMCID:
PMC4292751.



37. Blazevich AJ, Coleman DR, Horne S, Cannavan D. 2009; Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol. 105:869–78. DOI:
10.1007/s00421-008-0972-7. PMID:
19153760.


38. Trezise J, Blazevich AJ. 2019; Anatomical and neuromuscular determinants of strength change in previously untrained men following heavy strength training. Front Physiol. 10:1001. DOI:
10.3389/fphys.2019.01001. PMID:
31447693. PMCID:
PMC6691166.



39. Wang Y, Wluka AE, Berry PA, Siew T, Teichtahl AJ, Urquhart DM, Lloyd DG, Jones G, Cicuttini FM. 2012; Increase in vastus medialis cross-sectional area is associated with reduced pain, cartilage loss, and joint replacement risk in knee osteoarthritis. Arthritis Rheum. 64:3917–25. DOI:
10.1002/art.34681. PMID:
23192791.


40. Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. 2002; Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 61:617–22. DOI:
10.1136/ard.61.7.617. PMID:
12079903. PMCID:
PMC1754164.



41. Roos EM, Herzog W, Block JA, Bennell KL. 2011; Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 7:57–63. DOI:
10.1038/nrrheum.2010.195. PMID:
21119605.


42. Ruhdorfer A, Dannhauer T, Wirth W, Hitzl W, Kwoh CK, Guermazi A, Hunter DJ, Benichou O, Eckstein F. 2013; Thigh muscle cross-sectional areas and strength in advanced versus early painful osteoarthritis: an exploratory between-knee, within-person comparison in osteoarthritis initiative participants. Arthritis Care Res (Hoboken). 65:1034–42. DOI:
10.1002/acr.21965. PMID:
23401316.


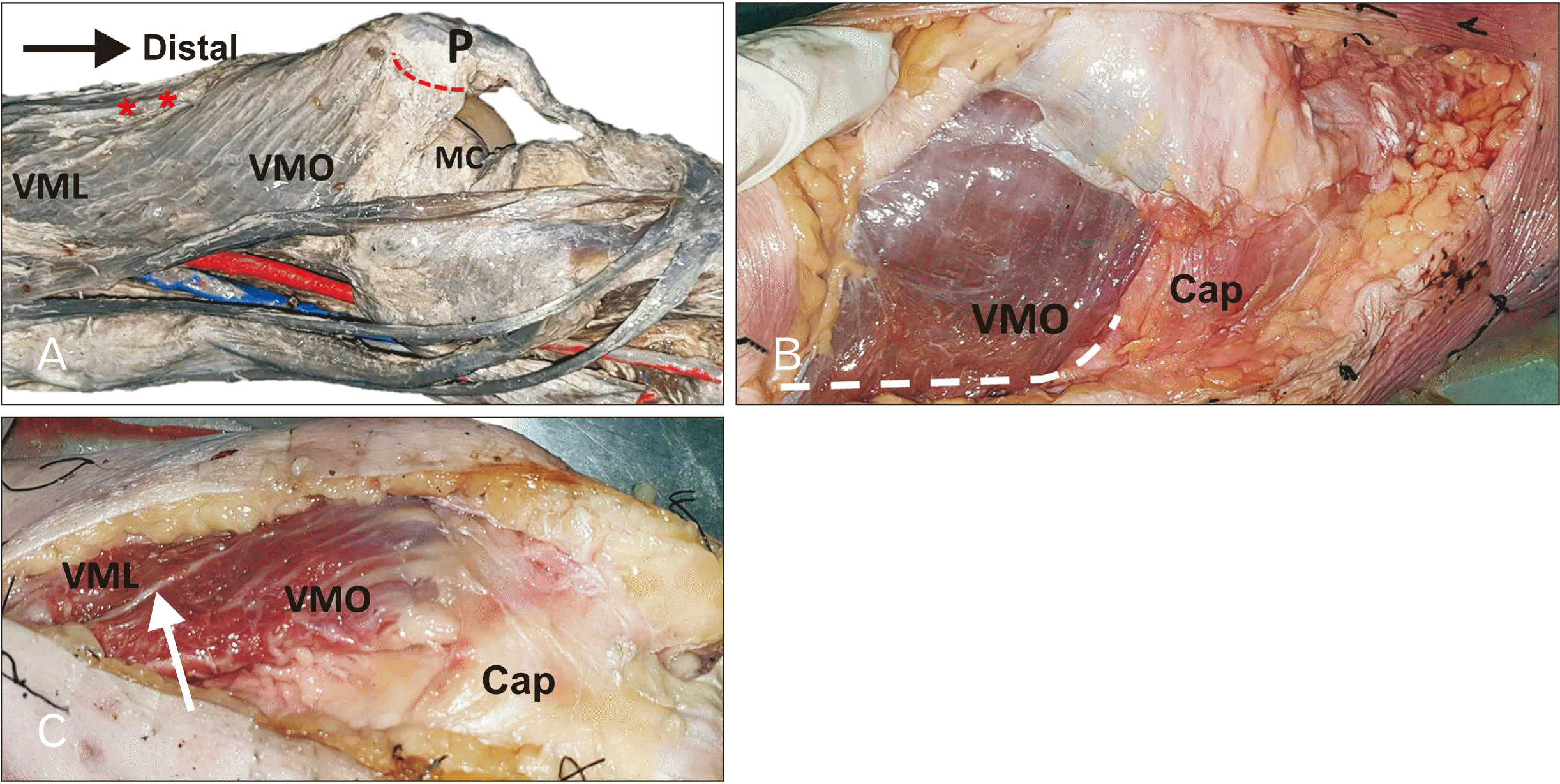
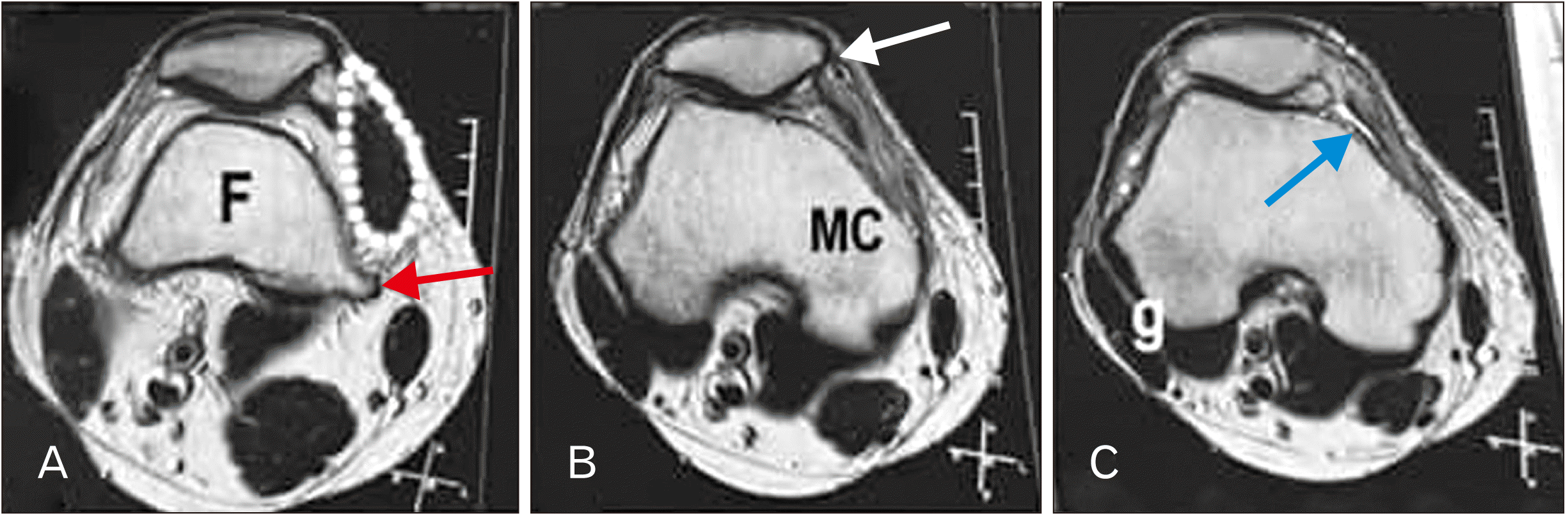
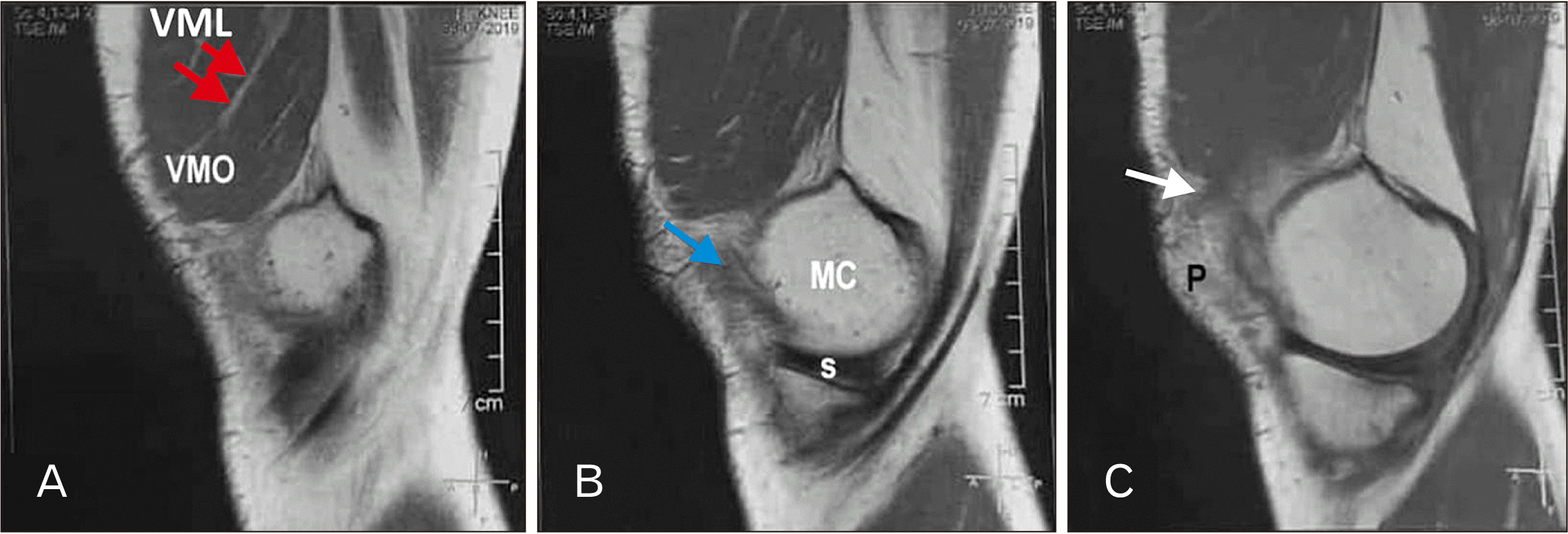
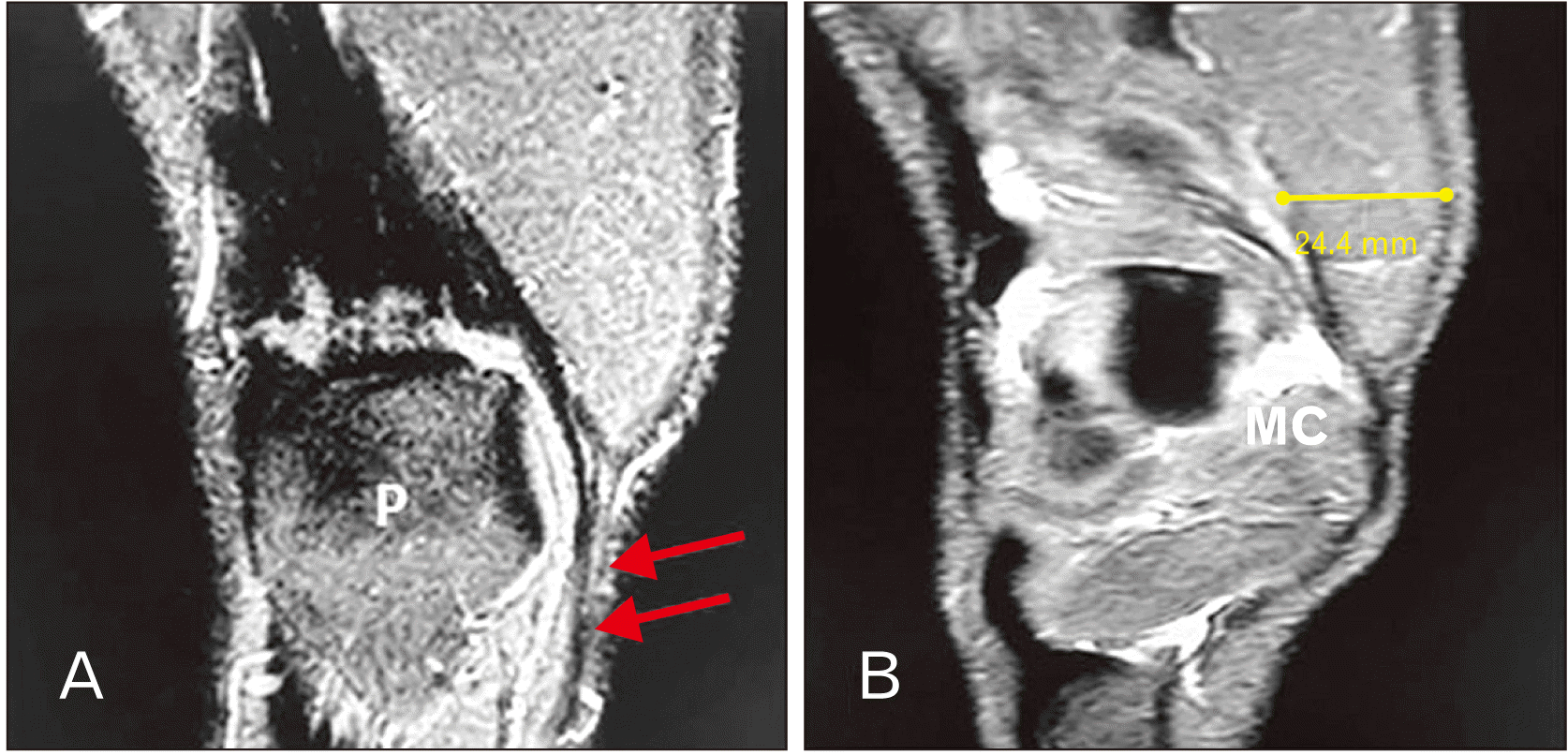




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download