INTRODUCTION
Since Lillehei et al.
1) attempted the total repair of tetralogy of Fallot (TOF), the operative mortality of TOF repair has decreased dramatically. However, as the long-term survival rate improved, postoperative morbidities associated with prior operations have been frequently observed. Those include right ventricular (RV) dilatation, irreversible RV failure, or lethal ventricular arrhythmia ultimately related with pulmonary regurgitation (PR) resulting from trans-annular reconstruction of the RV outflow tract (RVOT) in the previous operations.
2)3)4) Uncorrected PR can contribute to RV dysfunction, arrhythmias or sudden cardiac death after TOF repair.
2)3)4)5)6) In this situation, pulmonary valve replacement (PVR) timed correctly with adequate accompanying operations is the most effective treatment not only to prevent progression of RV dilation but also to restore dilated RV volume, to relieve heat failure symptoms, and ultimately to prevent sudden cardiac death from intractable ventricular arrhythmias.
7)8)9)10)
However, a significant portion of patients still complain of PR-related symptoms and signs despite having undergone PVR. There is ongoing debate about the optimal timing to perform PVR in patients with repaired TOF. Some studies have compared clinical outcomes between the matched patient groups and compare patients who underwent early PVR (before clinical symptoms were aggravated) and patients without PVR; these failed to prove significant superiority in the survival rate in patients who had undergone PVR
11)12) even though the patients who underwent PVR showed better survival.
Here, we reviewed the long-term clinical outcomes of patients undergoing TOF repair with trans-annular incision in terms of survival benefit of subsequent PVR. We also sought to determine the predictive factors of PVR after TOF repair with trans-annular incision.
Go to :

METHODS
Patients
This study was approved by the Institutional Review Board in Seoul National University Hospital (No. 1407-144-597, 1909-067-1064). The requirement for informed consent was waived because of the retrospective nature of the research. We identified 196 consecutive patients (126 males) who underwent TOF total repair with a trans-annular RVOT widening between January 1991 and December 1997. To analyze the effect of PVR on the long-term results of the patients, we included only survivors (n=186) after TOF repair, and then excluded 6 patients who underwent pulmonary valve repair. Eventually, 180 patients were enrolled in our analysis in this study. We excluded patients with other variant of RVOT, such as pulmonary atresia with major aortopulmonary collateral arteries or absent pulmonary valve syndrome, or patients who have other accompanying intracardiac defect, such as complete atrioventricular canal defect, for minimizing bias from different natural characteristics according to individual diagnoses. We limited the study period to evaluate long-term results for these patients’ groups. Our closing date of study was October 15, 2016.
Operation
We included patients who underwent trans-annular RVOT reconstruction. The details of this method have been described in a previous study.
13) Briefly, TOF repair was performed under moderate hypothermia (28–32°C) without circulatory arrest in most patients (n=188, 95.9%). The mean cardiopulmonary bypass time and mean aortic cross clamp time were 119±36 minutes and 63±22 minutes, respectively. Deep hypothermic circulatory arrest (DHCA) was used in 8 patients. The median DHCA time was 24 minutes. DHCA had been used in patients younger than 3 months of age. During the study period, the ventricular septal defect (VSD) was closed via a RV approach or a right atrial (RA) approach. Since 2002, we almost exclusively used a RA approach to close VSD. The decision to perform trans-annular RVOT widening was influenced by the z-score of the pulmonic valve annulus; however, this strategy was not clearly observed in the early study period (early 1990s) when the trans-annular RVOT widening had been chosen for almost all patients. If necessary, stenosis of pulmonary artery branch was repaired using a patch or a main pulmonary arterial flap.
Postoperative management and follow-up
Patients who underwent TOF repair were routinely checked with a 12-channel surface electrocardiogram, and a 2-dimensional (2D) echocardiography before discharge. If a patient had sustained a postoperative arrhythmia, then 24-hour Holter monitoring was considered. When the patient was stable after hospital discharge, we checked the clinical evaluation and imaging studies at 3- to 6-month intervals for 1 year at the outpatient clinic with mainly chest X-ray, and if needed, echocardiography. After 1 year, intervals of following-up were determined based on patient’s general status. Currently, most patients are regularly followed up immediately after cardiac surgery. If patients had heart failure symptoms, sustained arrhythmia, or RV dilatation revealed by 2D echocardiography, then cardiac magnetic resonance imaging (MRI) and a cardiopulmonary exercise test (CPET) were performed. The severity of PR was graded by echocardiography: none or trivial, mild, moderate, and severe.
About 83.3% (n=150, out of 180) of patients had postoperative echocardiographic reports at median 20.3 years after operation (range, 10.5–25.2 years). Some data recorded in the paper were lost during our digitalization processing for electric medical record system. If patients were suspected to being deceased (did not appear in the outpatient clinic for a long time or not being reached by telephone contact), then their mortality was confirmed from the Korean National Health Insurance Service system. Regarding the data associated with survival, we checked all patients' status from National Health Insurance Service system. By October 15, 2016, 135 patients (75%) were followed-up within recent 5 years, and 24 patients (13.3%) within 5 to 10 years. Twenty-one patients (11.7%) did not visited the hospital within recent 10 years.
An early death was defined as mortality during hospitalization for total repair or within 30 days after total repair and late death was defined as all-cause mortality. Event free survival was defined as survival free from a composite of death, arrhythmia, reoperation for PR and neurological complications including stroke, intracranial hemorrhage, and seizure.
Cardiac MRI was usually performed when the 2D echocardiography revealed severe RV dilatation with a moderate to severe PR. A CPET was also performed at the discretion of the referring cardiologist if required. We recently checked the CPET in almost patients who underwent TOF total correction. During follow-up, 70 patients (38.3%) underwent postoperative cardiac MRI at median 19.4 years (IQR, 15.8–22.3 years) and 112 patients (61.2%) had a CPET performed at median 20.2 years (IQR, 18.4–22.8 years) after TOF correction.
Subgroup analysis (pulmonary valve replacement vs. non-pulmonary valve replacement)
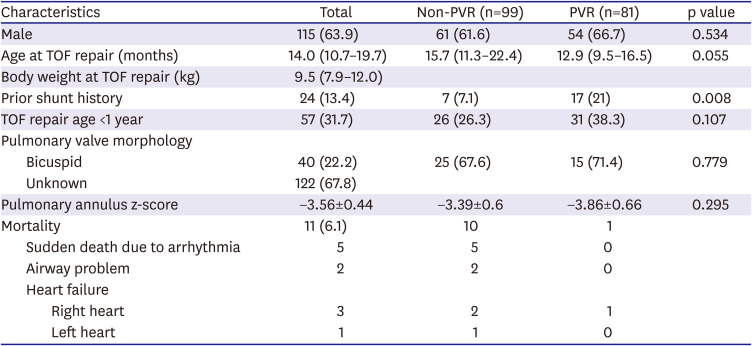
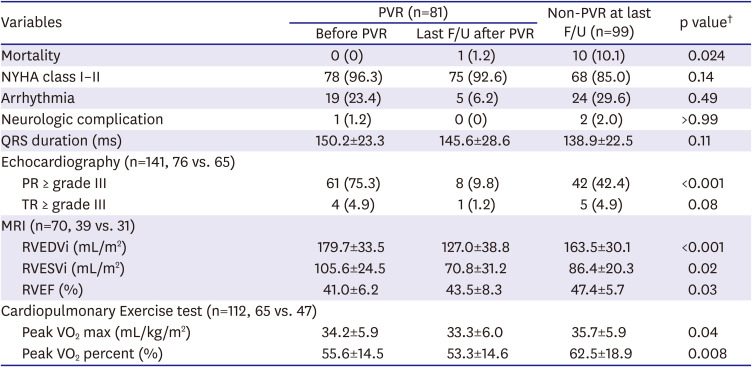
During the study period, the decision to perform PVR was usually affected by clinical situations such as aggravated tricuspid regurgitation (TR), arrhythmia, or other RV failure symptoms. Although a cardiac MRI was not routinely performed in the study period, if a patient had a MRI examination, a RV end-diastolic volume index (RVEDVi) was accepted as one of important parameters to determine PVR; particularly, when RVEDVi was more than 170 mL/m2, PVR was considered at the time. In-hospital deaths after TOF repair (n=10) and cases of pulmonic valve repair during follow-up (n=6) were excluded to compare long-term survival benefits of patients with or without PVR. We excluded the patients who underwent valve repair because repair is often not as effective as eliminating valve regurgitation as replacement. Therefore, 180 patients (PVR group: n=81; non-PVR group: n=99) were enrolled for subgroup analysis.
Statistical analysis
Categorical variables expressed as frequencies or percentages and continuous variables are presented as the mean±standard deviation or the median with a range. If the data were normally distributed, then we used a parametrical test such as χ2, t-test, or paired t-test. If not, we used non-parametrical tests such as Fisher's exact test, Mann-Whitney U test, or Wilcoxon's signed rank test. Overall follow-up duration was calculated as an interval from the day when the TOF operation was performed to the day when the last follow-up was made. For cases of mortality, the day when the patient died was considered to be the last follow-up day. A Cox regression model was fitted to identify predictive factors associated with PVR after TOF repair. For subgroup analyses, survival curves were constructed using Kaplan-Meier estimates and compared with the log-rank test. All reported p values were 2-sided, and a p value (p) less than 0.05 was considered to denote a statistically significant difference. SPSS (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Go to :

DISCUSSION
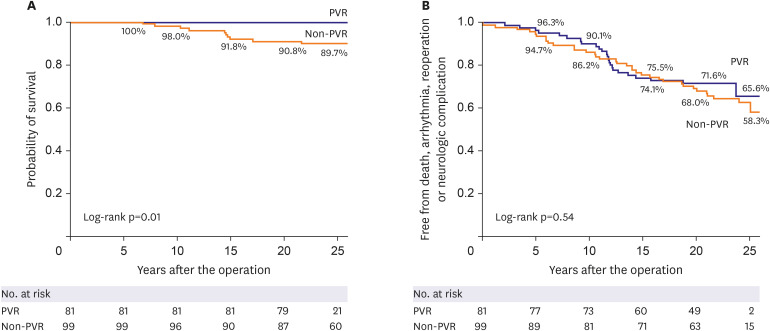
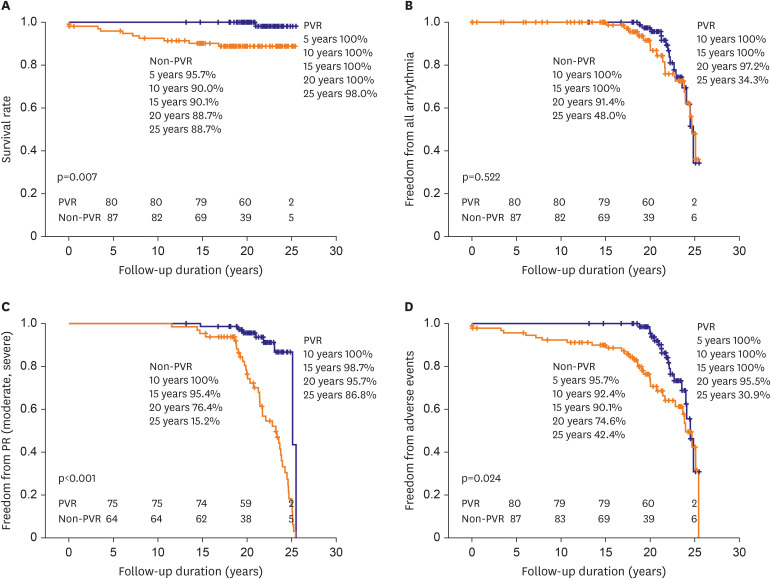
Our results show that patients undergoing TOF repair with trans-annular incision have acceptable long-term outcomes with several morbidities associated with postoperative PR, arrhythmia, and RV dilatation. The patients who underwent PVR during the follow up showed better survival rate than the patients who did not.
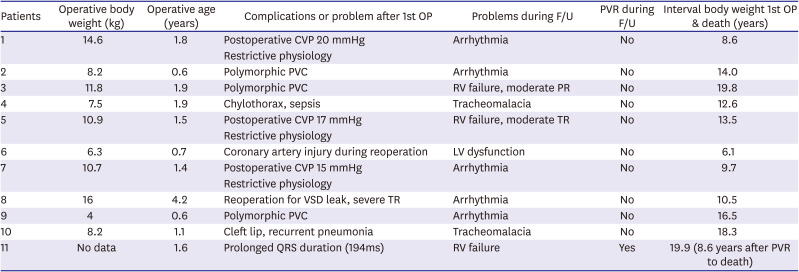
The late survival was significantly worse in patients with a sustained arrhythmia or RV dilatation. There were 11 late deaths (5.6%) after TOF repair, and 5 of them seemed to be sudden deaths. These 5 patients with had a temporary change in rhythm due to ventricular arrhythmia. Among the other 6 patients, 2 patients in the non-PVR group suffered from RV dysfunction accompanying moderate PR and TR, and 1 patient in the PVR group presented New York Heart Association (NYHA) class III of dyspnea due to irreversible RV dysfunction even after PVR. The RVEDVi of this patient was 233 mL/m
2 on cardiac MRI before PVR. This was performed at 11 years after TOF repair, which was rather shorter interval between TOF repair and PVR than median interval of patients in the PVR group (12.2 years). Despite shorter interval, an irreversible RV dysfunction with severe dilatation already occurred in this patient. Regarding the mortality in non-PVR patients, among 10 patients in non-PVR group, 3 cases seemed not directly to be related with RV problems (airway problem in 2, LV dysfunction due to coronary injury in 1) and 7 mortalities were related with RV failure or arrhythmia related with RV failure (
Table 2). For these patients, if we had performed a scheduled pulmonary valve implantation earlier, we could've prevented mortalities in some of those patients with RV failure accompanying with tricuspid valvular problems; however, presumably we could not predict sudden death that might be related with arrhythmia.
Previous studies showed that a trans-annular incision tends to result in progression of PR over time late after TOF repair.
2)3)4)5)6)14) Geva et al.
5) evaluated the factors associated with impaired clinical status in 100 long-term survivors of TOF repair evaluated by MRI and clearly demonstrated that free PR had a negative effect on RV systolic function and clinical status. Kirklin et al.
15) performed one of the largest retrospective studies to date and evaluated the outcome of 814 patients undergoing TOF repair with transannular patching. They showed much more severe adverse composite outcomes of all-cause mortality and clinical status. To address these possible drawbacks, the current surgical strategy of TOF in our center includes early primary repair with preservation of pulmonary valve.
16)17)18)
Our study revealed that 94 (50.5%) patients showed moderate to severe PR on their most recent echocardiography. Most of these patients (81patients, 43.5%) underwent PVR throughout the entire study period. This result is comparable to the incidence of reintervention for PR after TOF repair in a contemporary series (14.1–75%).
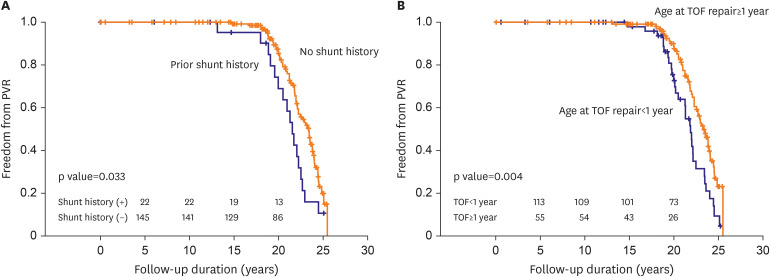
19)20) The predictive factors for receiving PVR during long-term follow up are being younger than 1-year-old at the time of TOF repair and prior shunt history. The patients who underwent TOF correction with trans-annular RVOT widening under 1 year old must had been exposed to PR longer than the patients who underwent total correction older than 1 year of age. These results were also consistent with findings from previous studies.
4)21)22) Kogon et al.
21) reported that age at the time of PV disruption was significantly associated with PVR. Gatzoulis et al.
4) also showed that surrogates of increased pulmonary vascular resistance (prior shunt history before TOF repair) or volume load were also associated with increased adverse effects of PR.
A small pulmonary valve annulus (z-score <−3) was also expected to be a risk factor for PVR. However, it was not a risk factor for PVR in our study (HR, 0.86; CI, 0.54–1.38; p=0.53) because most patients in this study period underwent transannular RVOT widening regardless of size of pulmonary valve annulus. The small size of pulmonary annulus might have been a risk factor for early PVR if we had tried to preserve pulmonary valve annulus more aggressively for the patients with −3 or −4 of pulmonary valve annulus z-score in this era as we are doing now in our institution.
18)
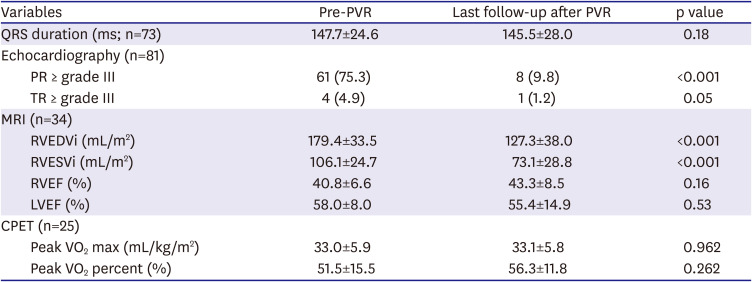
Even though PVR was performed relatively late in our study versus with current trends, patients with PVR still showed a significant reduction in RV volume after PVR. The RVEF had a tendency toward improvement that did not reach statistical significance. This finding might be explained by exclusion of pre-operative aneurysmal area in the infundibulum by PVR.
Considering the interval between TOF operation and last follow-up in non-PVR group (median, 19.3 years), it was definitely longer than the interval from TOF operation to 1st PVR operation in the PVR group (median, 12.2 years). Although we do not have enough matched data from cardiac MRI, the patients in the non-PVR showed smaller RVEDVi (163.5 mL/m
2) than the patients (179.7 mL/m
2) in the PVR-group before PVR (
Table 4), despite longer follow-up duration. RVEF of the patients in non-PVR was also better (47.4%) than that of PVR patients (41.0%) before PVR (
Table 4). Also, the ratio of the patients showing more than moderate degree of PR was higher in the PVR group before PVR was done. Recently, our indications for PVR have become more complicated and recommend little bit earlier PVR than before, in this study, these non-PVR patients did not meet the indications at the time. Therefore, we could assume that PVR patients were in worse clinical situations before PVR than the patients in non-PVR group even in the young age, 14.4±4.3 years, before PVR, and despite that, they significantly showed better outcomes in survival rate, adverse events after PVR comparing with the non-PVR patients. Although the non-PVR patients' symptoms, signs or some parameters from examination did not meet the indications for PVR despite longer follow-up, but their clinical outcomes were not satisfactory.
Sudden death occurred in 5 patients who did not have PVR, but none of the PVR subjects had sudden death during follow-up (
Figure 3). This implies that PVR after TOF repair may have a beneficial effect on long-term survival in contrast to some recent suggestions from other matched cohort studies that there is no clear long-term survival benefit after PVR.
11)12) The discrepancies in results between our study and the above studies might be due to different indications for the timing of PVR.
Debates persists regarding PVR indications and timings. However, some studies suggest that PVR should be considered early to obtain optimal long-term outcomes when the patients' RV is dilated.
23)24) In our studies, a RVEDVi more than 170 mL/m
2 on cardiac MRI was one of the major determinants for pulmonary valve implantation for PR after TOF repair in patients without signs and symptoms of RV failure. We found that patients actually underwent PVR when preoperative RVEDVi was 179.4±33.5 mL/m
2 in this study. In terms of RV volume aspects, timing of PVR in this study seemed relatively delayed when we compared it with several previous studies
23)24) and even with our own current strategy. We assumed that this belated timing of PVR was associated with some of the observed late deaths of the patients with RV dilatation in our study. Interestingly, most patients with dilated RV did not complain of significant clinical symptoms during follow-up at the out-patients clinic. At their most recent follow-up, 81.7% of patients (157/186 survivors) showed a NYHA functional status class I or II. These features mattered because further evaluation such as cardiac MRI might be delayed or not even considered in these patients until the patients showed significant heart failure signs. This trend could be attributed to belated PVR that would be associated with a high mortality rate in patients with significant PR during follow-up. Related to this issue, since 2003, we included a cardiac MRI as a routine evaluation tool for every repaired TOF patient. We currently recommend a PVR when the patients show significant clinical symptoms and signs associated with RV failure. For asymptomatic patients, we consider a PVR when the RVEDVi from cardiac MRI is more than 150-160 mL/m
2 during follow-up. We then, check data from echocardiography, cardiac MRI, 24-hour Holter monitoring, and cardiac catheterization more frequently than the usual regular intervals. If these parameters worsen, then we recommend a PVR to the patients and patients’ family even the patient does not show significant clinical symptoms or signs.
This study is a retrospective review from a single center. Incomplete data sets might affect the statistical power for analyses. We reviewed archival data (from 1991 to 1997), and some data were missed or not completely measured. In addition to this, some parts of the hand-written data were not preserved during digitalization. The useful data of cardiac MRI and CPET test for comparison between each group were only available in 70 and 111 patients, respectively, because we did not check these examinations routinely in the early study period. These insufficiency of matched data and missing hospital records made us difficult to perform a matching analyses between 2 groups, PVR and non-PVR group. However, if we had performed a matching analyses, the non-PVR patients, who have followed-up a longer duration in clinically tolerable status, might have been left out in analysis and their important data also might have not been included. Since 2004, we have scanned most of the patients. One thing we have to mention is that we have modified our treatment strategies for this disease based on our experiences including the results from this study, therefore, there are some discrepancies between current treatment strategies and previous treatment strategies that described in this research.
Another limitation of this study is that following-up of some patients was not made regularly, and 11% of patients did not visit the hospital for more than recent 10 years. For these patients, we had to use their most recent data although they were old.
In conclusion, we confirmed that arrhythmias were frequently observed after repair of TOF with trans-annular incision during follow-up. The patient age at the time of TOF repair and the history of shunt implantation before repair of TOF were predictive factors for requiring PVR. The early, adequate timing of PVR could be beneficial for long-term survival in the patients who have significant PR after TOF repair with trans-annular RVOT widening manner because the patients who had been exposed to significant PR without PVR for a long time showed a significantly higher rate of mortality.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download