1. Kim Y, Ahn Y, Cho MC, Kim CJ, Kim YJ, Jeong MH. Current status of acute myocardial infarction in Korea. Korean J Intern Med. 2019; 34:1–10. PMID:
30612415.

2. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39:119–177. PMID:
28886621.

3. Boersma E. Primary Coronary Angioplasty vs. Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006; 27:779–788. PMID:
16513663.
4. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994; 343:311–322. PMID:
7905143.

5. Pinto DS, Frederick PD, Chakrabarti AK, et al. Benefit of transferring ST-segment-elevation myocardial infarction patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase. Circulation. 2011; 124:2512–2521. PMID:
22064592.

6. Gershlick AH, Stephens-Lloyd A, Hughes S, et al. Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med. 2005; 353:2758–2768. PMID:
16382062.

7. Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta-analysis. Eur Heart J. 2010; 31:2156–2169. PMID:
20601393.

8. D'Souza SP, Mamas MA, Fraser DG, Fath-Ordoubadi F. Routine early coronary angioplasty versus ischaemia-guided angioplasty after thrombolysis in acute ST-elevation myocardial infarction: a meta-analysis. Eur Heart J. 2011; 32:972–982. PMID:
21036776.

9. Madan M, Halvorsen S, Di Mario C, et al. Relationship between time to invasive assessment and clinical outcomes of patients undergoing an early invasive strategy after fibrinolysis for ST-segment elevation myocardial infarction: a patient-level analysis of the randomized early routine invasive clinical trials. JACC Cardiovasc Interv. 2015; 8:166–174. PMID:
25616922.

10. Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009; 360:2705–2718. PMID:
19553646.

11. Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013; 368:1379–1387. PMID:
23473396.

12. Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006; 355:2395–2407. PMID:
17105759.


13. Park J, Choi KH, Lee JM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST-segment-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc. 2019; 8:e012188. PMID:
31041869.
14. Sim DS, Jeong MH, Ahn Y, et al. Pharmacoinvasive strategy versus primary percutaneous coronary intervention in patients with ST-segment-elevation myocardial infarction: a propensity score-matched analysis. Circ Cardiovasc Interv. 2016; 9:e003508. PMID:
27582112.
15. Nordmann AJ, Hengstler P, Harr T, Young J, Bucher HC. Clinical outcomes of primary stenting versus balloon angioplasty in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Am J Med. 2004; 116:253–262. PMID:
14969654.
16. Stone GW, Grines CL, Cox DA, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002; 346:957–966. PMID:
11919304.

17. Kastrati A, Dibra A, Spaulding C, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007; 28:2706–2713. PMID:
17901079.

18. Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012; 380:1482–1490. PMID:
22951305.

19. Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016; 387:357–366. PMID:
26520230.

20. Kelbæk H, Høfsten DE, Køber L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet. 2016; 387:2199–2206. PMID:
27053444.

21. Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015; 385:2465–2476. PMID:
25791214.

22. Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012; 60:2481–2489. PMID:
22858390.
23. Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013; 369:1587–1597. PMID:
23991656.

24. Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015; 372:1389–1398. PMID:
25853743.


25. Jolly SS, Cairns JA, Yusuf S, et al. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. 2015; 36:2364–2372. PMID:
26129947.


26. Jolly SS, James S, Džavík V, et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: Thrombectomy Trialists Collaboration. Circulation. 2017; 135:143–152. PMID:
27941066.

27. Ellis SG, Tendera M, de Belder MA, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008; 358:2205–2217. PMID:
18499565.

28. Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008; 358:2218–2230. PMID:
18499566.

29. Park KW, Kang SH, Chung WY, et al. ‘Real world’ comparison of drug-eluting stents vs bare metal stents in the treatment of unselected patients with acute ST-segment elevation myocardial infarction. Circ J. 2010; 74:1111–1120. PMID:
20453391.

30. Sim DS, Jeong MH, Ahn Y, et al. Effectiveness of drug-eluting stents versus bare-metal stents in large coronary arteries in patients with acute myocardial infarction. J Korean Med Sci. 2011; 26:521–527. PMID:
21468259.


31. Piao ZH, Jeong MH, Li Y, et al. Comparison of second-generation drug-eluting versus bare-metal stents in octogenarian patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2014; 177:1081–1084. PMID:
25456703.

32. Kim JS, Lee HJ, Woong Yu C, et al. INNOVATION study (Impact of Immediate Stent Implantation Versus Deferred Stent Implantation on Infarct Size and Microvascular Perfusion in Patients With ST-Segment-Elevation Myocardial Infarction). Circ Cardiovasc Interv. 2016; 9:e004101. PMID:
27965296.
33. Kim N, Lee JH, Jang SY, et al. Radial versus femoral access with or without vascular closure device in patients with acute myocardial infarction. Am J Cardiol. 2019; 123:742–749. PMID:
30563616.

34. Li H, Rha SW, Choi BG, et al. Transradial versus transfemoral intervention in ST-segment elevation myocardial infarction patients in Korean population. Korean J Intern Med. 2018; 33:716–726. PMID:
28859467.

35. Sim DS, Jeong MH, Ahn Y, et al. Manual thrombus aspiration during primary percutaneous coronary intervention: impact of total ischemic time. J Cardiol. 2017; 69:428–435. PMID:
26867778.

36. Hachinohe D, Jeong MH, Saito S, et al. Clinical impact of thrombus aspiration during primary percutaneous coronary intervention: results from Korea Acute Myocardial Infarction Registry. J Cardiol. 2012; 59:249–257. PMID:
22341434.

37. Kim JS, Park SM, Kim BK, et al. Efficacy of clotinab in acute myocardial infarction trial-ST elevation myocardial infarction (ECLAT-STEMI). Circ J. 2012; 76:405–413. PMID:
22146757.

38. Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006; 48:1319–1325. PMID:
17010789.

39. O'Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008; 300:71–80. PMID:
18594042.

40. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020; [Epub ahead of print].
41. Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009; 360:2165–2175. PMID:
19458363.

42. Jobs A, Mehta SR, Montalescot G, et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: a meta-analysis of randomised trials. Lancet. 2017; 390:737–746. PMID:
28778541.

43. Kofoed KF, Kelbæk H, Hansen PR, et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome. Circulation. 2018; 138:2741–2750. PMID:
30565996.

44. Sim DS, Jeong MH, Ahn Y, et al. Clinical impact of immediate invasive strategy in patients with non-ST-segment elevation myocardial infarction. Int J Cardiol. 2016; 221:937–943. PMID:
27441472.

45. Kim MC, Jeong MH, Sim DS, et al. Optimal timing of percutaneous coronary intervention in patients with non-ST-segment elevation myocardial infarction complicated by acute decompensated heart failure (from the Korea Acute Myocardial Infarction Registry-National Institutes of Health [KAMIR-NIH]). Am J Cardiol. 2018; 121:1285–1292. PMID:
29680172.

46. Jaski BE, Cohen JD, Trausch J, et al. Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single-vessel versus multivessel coronary artery disease. Am Heart J. 1992; 124:1427–1433. PMID:
1462895.

47. Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007; 28:1709–1716. PMID:
17556348.

48. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:e362–425. PMID:
23247304.
49. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011; 124:e574–e651. PMID:
22064601.
50. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016; 133:1135–1147. PMID:
26490017.

51. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013; 369:1115–1123. PMID:
23991625.

52. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015; 65:963–972. PMID:
25766941.


53. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015; 386:665–671. PMID:
26347918.

54. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017; 376:1234–1244. PMID:
28317428.

55. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019; 381:1411–1421. PMID:
31475795.

56. Sardella G, Lucisano L, Garbo R, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016; 67:264–272. PMID:
26796390.

57. Kwon SW, Park SD, Moon J, et al. Complete versus culprit-only revascularization for ST-segment elevation myocardial infarction and multivessel disease in the 2nd generation drug-eluting stent era: data from the INTERSTELLAR registry. Korean Circ J. 2018; 48:989–999. PMID:
30334385.


58. Ahn MJ, Kim MC, Ahn Y, et al. Impact of complete revascularization on six-year clinical outcomes and incidence of acute decompensated heart failure in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease. Am J Cardiol. 2018; 121:544–551. PMID:
29325904.

59. Kim MC, Jeong MH, Ahn Y, et al. What is optimal revascularization strategy in patients with multivessel coronary artery disease in non-ST-elevation myocardial infarction? Multivessel or culprit-only revascularization. Int J Cardiol. 2011; 153:148–153. PMID:
20843572.

60. Ahn KT, Oh JK, Seong SW, et al. One-year clinical outcomes between single- versus multi-staged PCI for ST elevation myocardial infarction with multi-vessel coronary artery disease: from Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH). Korean Circ J. 2020; 50:220–233. PMID:
32100479.

61. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID:
30165437.

62. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017; 377:2419–2432. PMID:
29083953.

63. Yang JH, Hahn JY, Song PS, et al. Percutaneous coronary intervention for nonculprit vessels in cardiogenic shock complicating ST-segment elevation acute myocardial infarction. Crit Care Med. 2014; 42:17–25. PMID:
24105454.

64. Lee JM, Rhee TM, Hahn JY, et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2018; 71:844–856. PMID:
29471935.

65. Lee JM, Rhee TM, Kim HK, et al. Comparison of long-term clinical outcome between multivessel percutaneous coronary intervention versus infarct-related artery-only revascularization for patients with ST-segment-elevation myocardial infarction with cardiogenic shock. J Am Heart Assoc. 2019; 8:e013870. PMID:
31818215.
66. Thiele H, Akin I, Sandri M, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018; 379:1699–1710. PMID:
30145971.

67. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010; 362:2155–2165. PMID:
20558366.

68. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012; 367:1287–1296. PMID:
22920912.

69. Thiele H, Zeymer U, Thelemann N, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2018; [Epub ahead of print].
70. Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017; 69:278–287. PMID:
27810347.

71. Kim HK, Jeong MH, Ahn Y, et al. Clinical outcomes of the intra-aortic balloon pump for resuscitated patients with acute myocardial infarction complicated by cardiac arrest. J Cardiol. 2016; 67:57–63. PMID:
25982668.

72. Kim H, Lim SH, Hong J, et al. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation. 2012; 83:971–975. PMID:
22322287.

73. Choi KH, Yang JH, Park TK, et al. Risk prediction model of in-hospital mortality in patients with myocardial infarction treated with venoarterial extracorporeal membrane oxygenation. Rev Esp Cardiol (Engl Ed). 2019; 72:724–731. PMID:
30037538.

74. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018; 138:e618–e651. PMID:
30571511.
75. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015; 131:861–870. PMID:
25587100.

76. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017; 135:1481–1489. PMID:
28179398.

77. Kang WY, Jeong MH, Ahn YK, et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011; 146:207–212. PMID:
19664828.

78. Choo EH, Chang K, Lee KY, et al. Prognosis and predictors of mortality in patients suffering myocardial infarction with non-obstructive coronary arteries. J Am Heart Assoc. 2019; 8:e011990. PMID:
31284804.
79. Baek JY, Choi BG, Rha SW, et al. Comparison of two-year outcomes of acute myocardial infarction caused by coronary artery spasm versus that caused by coronary atherosclerosis. Am J Cardiol. 2019; 124:1493–1500. PMID:
31547996.

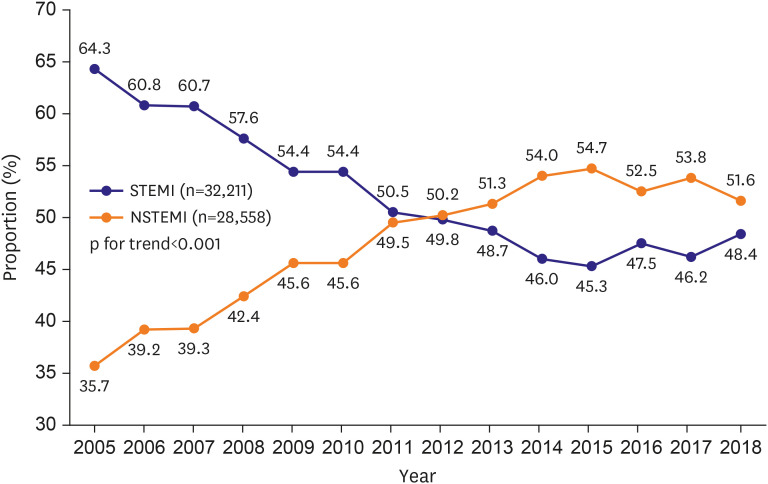
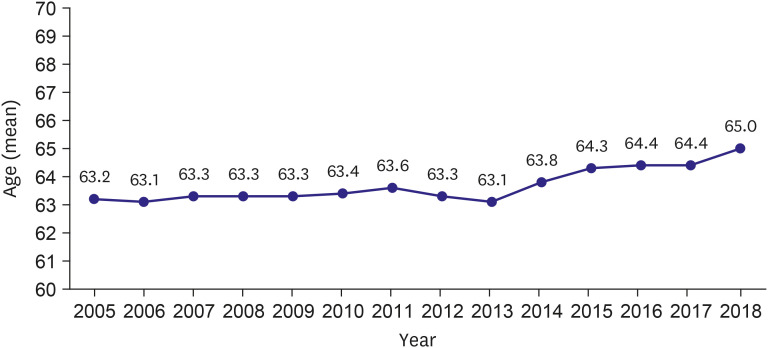
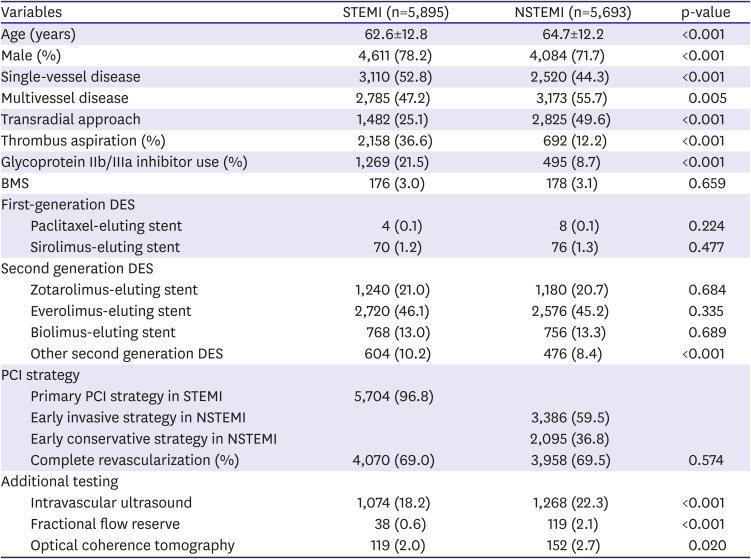
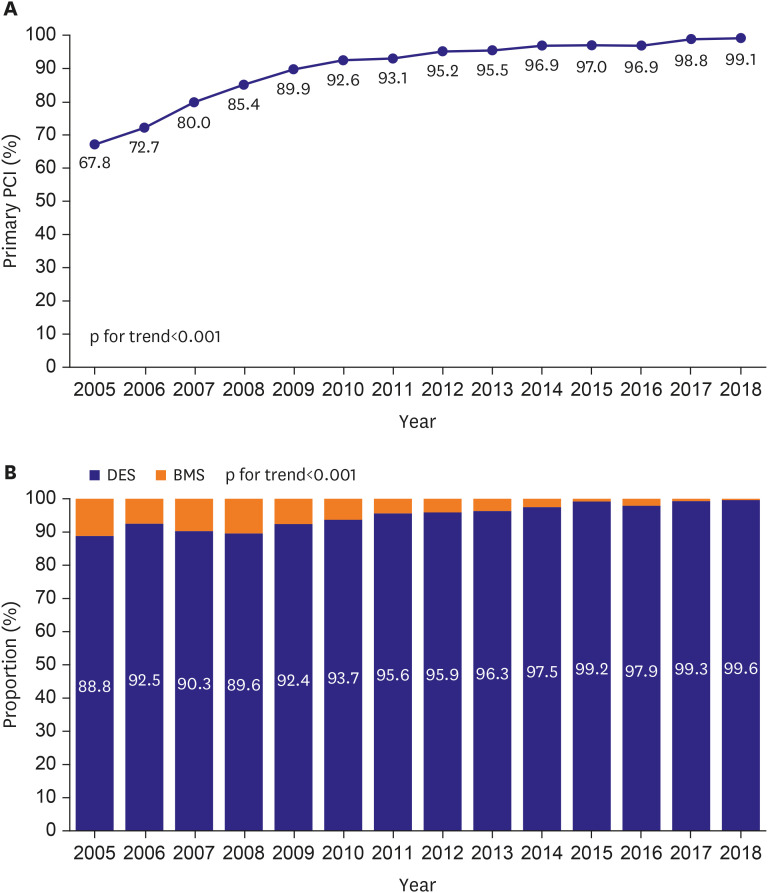
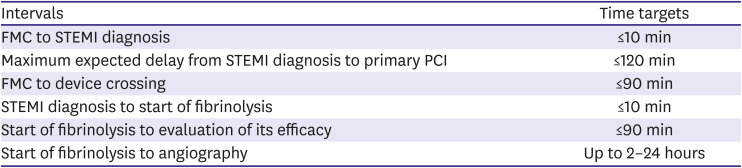
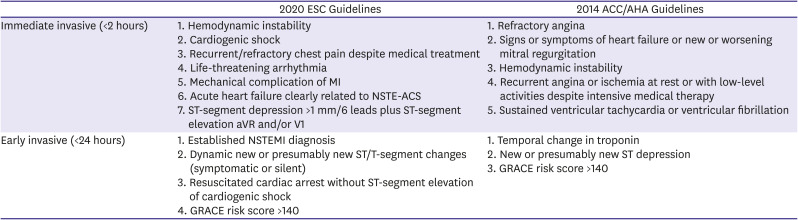
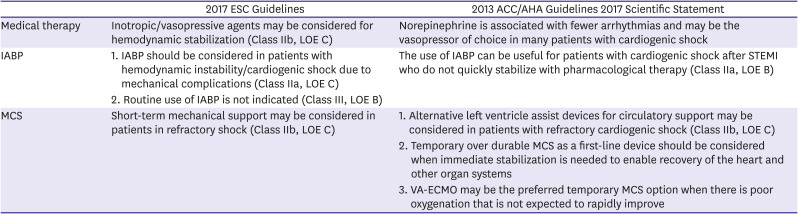




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download