TO THE EDITOR: Unlike primary myelofibrosis (PMF), acute myelofibrosis (AMF) is a distinct clinicopathological entity characterized by the sudden onset of pancytopenia, extensive bone marrow (BM) fibrosis, megakaryocytic hyperplasia with or without dysplasia, leukoerythroblastic blood picture, and absence of hepatosplenomegaly (HSM) and no tear drop cells [1-8]. AMF is an uncommon presentation of acute myeloid leukemia (AML; particularly AML-M7), acute panmyelosis with myelofibrosis, and occasionally myeloproliferative neoplasm [especially chronic myeloid leukemia (CML)] [9, 10]. BM fibrosis has been reported in acute lymphoblastic leukemia (ALL) at diagnosis (B cell-ALL>T cell-ALL); although BM fibrosis has been shown to correlate with a low minimal residual disease (MRD)-negative rate at the end-of-induction (EOI) [11], AMF in association with ALL is extremely rare. AMF may be either concurrent with ALL or precede its onset. We present two cases of ALL that were preceded by AMF and a literature review.
Go to : 
Case 1
A 50-year-old man presented to our hospital in June 2018 with a two-month history of progressive fatigue. Past medical history was significant for diabetes mellitus, which was well controlled with antidiabetic medications. Examination revealed marked pallor and no HSM or lymphadenopathy. Complete hemogram revealed pancytopenia (hemoglobin, 6 g/dL; white cell counts, 3.25×109/L; and platelets, 78×109/L) without any atypical cells. BM aspiration (BMA) performed at an outside hospital in May 2018 was a dry tap. BM biopsy revealed extensive reticulin fibrosis [World Health Organization (WHO) grade 2] without any blasts (Fig. 1). He was managed symptomatically with blood transfusions. In July 2018, peripheral blood (PB) examination showed a left shift with 13% blasts. He did not have eosinophilia or basophilia. Moreover, BMA performed in July 2018 was a dry tap. BM biopsy revealed dense reticulin fibrosis (WHO grade 2) admixed with blasts. Findings of PB flow cytometry (FCM) were consistent with the diagnosis of precursor B-cell ALL (Pre-B ALL; Fig. 2A). BM cytogenetics could not be performed due to dry tap. Reverse transcription polymerase chain reaction (RT-PCR) using PB was positive for the BCR-ABL transcript (p190), whereas JAK-2, CALR, and MPL mutations were negative. He was treated with the European Working Group for Adult ALL (EWALL) protocol for Philadelphia-positive ALL (Ph+-ALL) along with imatinib. EOI BMA and biopsy (day-33) were in morphological remission, with significant resolution of BM fibrosis (WHO grade 0–1). Complete molecular remission was achieved at 3 months. However, the disease relapsed after 1 year, and he died 2 months after relapse.
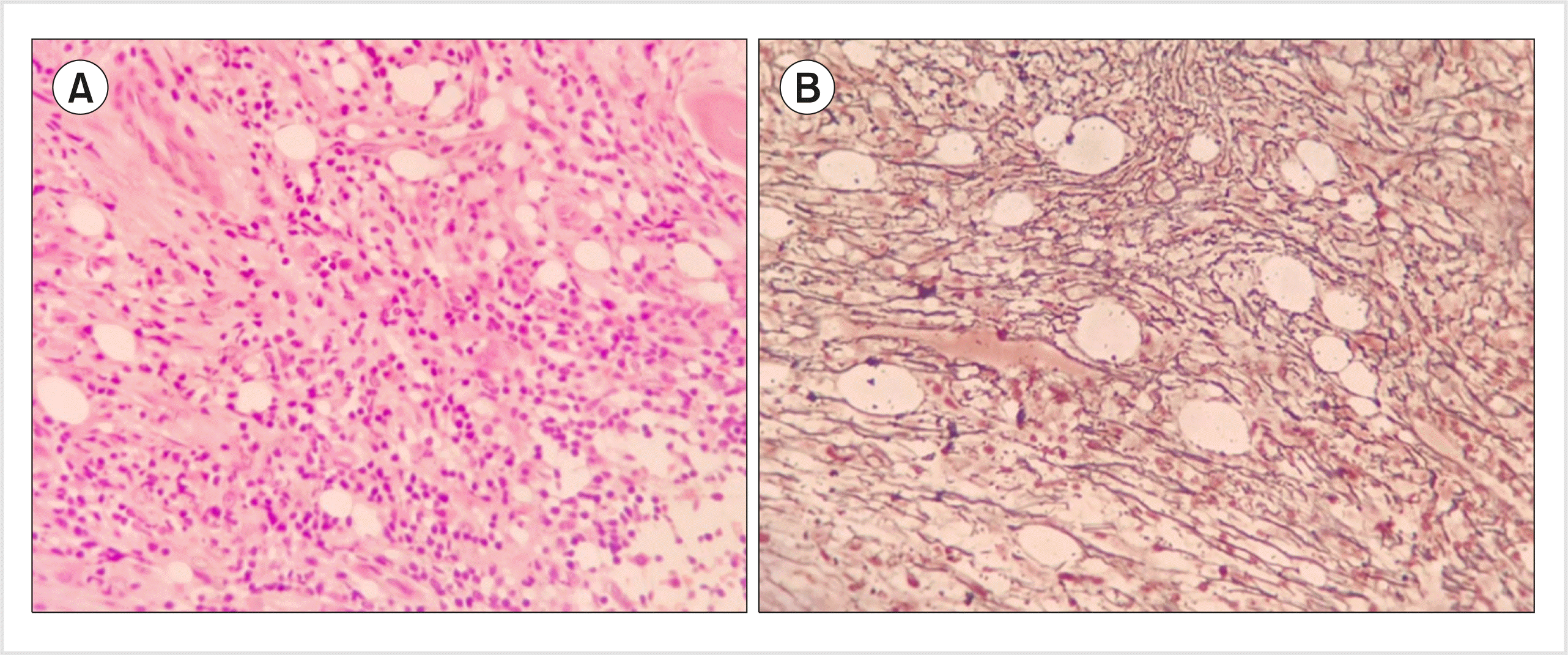 | Fig. 1Bone marrow biopsy. (A) Extensive fibrosis (WHO grade: 2) with interspersed atypical cells (hematoxylin and eosin, ×10). (B) Reticulin fibrosis (silver stain, ×10). |
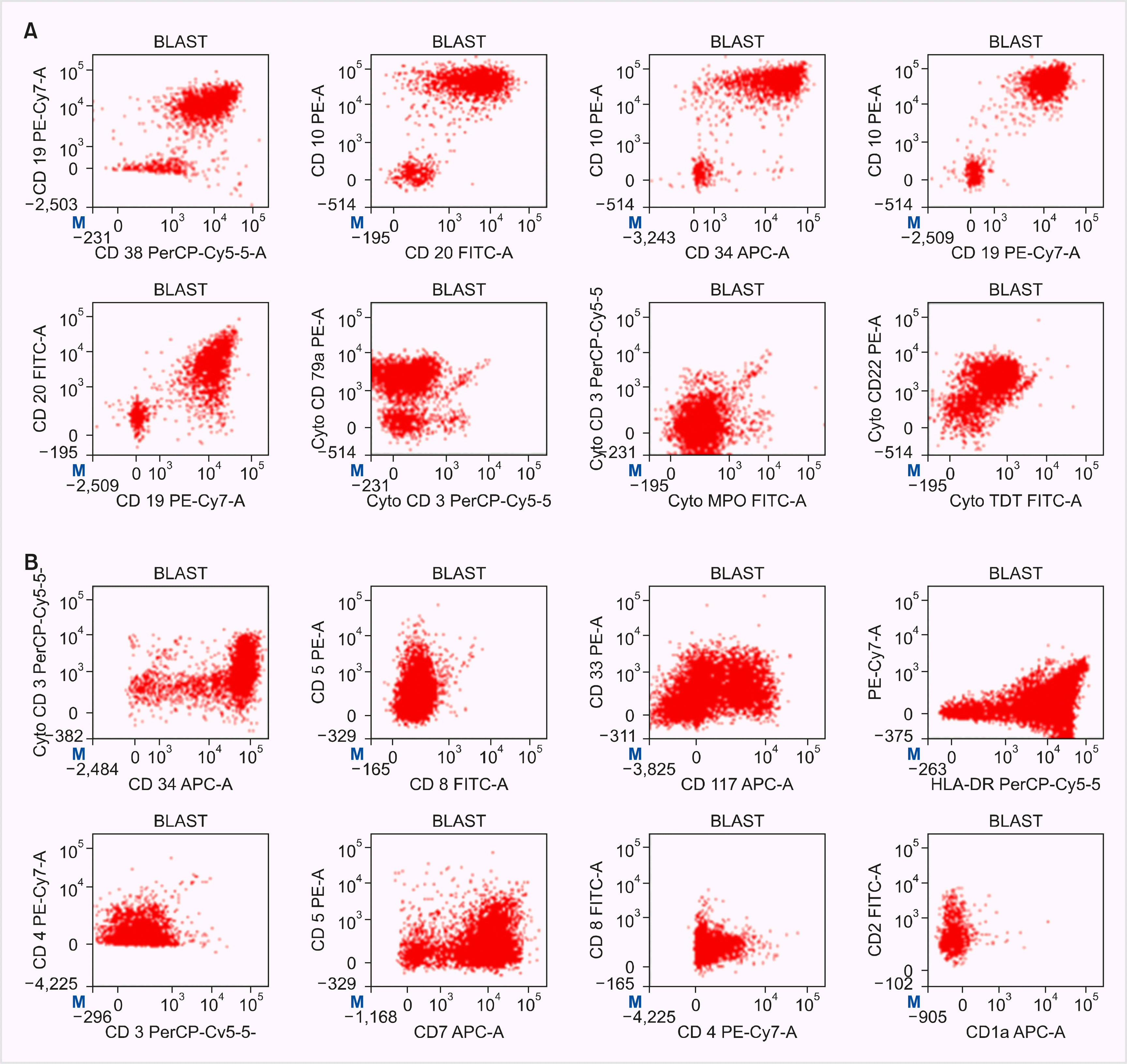 | Fig. 2Flow cytometry plots. (A) Precursor B-ALL. Blasts were positive for CD34, CD117, CD38, C20, CD19, CD79a, CD10, and cytoplasmic CD22. Blasts were negative for HLADR, cytoplasmic CD3, CD7, CD5, CD1a, CD2, CD3, CD4, CD8, CD13, CD33, MPO, CD64, and CD10. (B) Early T-precursor ALL. Blasts were positive for CD34, CD117, HLADR, cCD3, CD38, CD123, CD99, and CD7. Blast were negative for CD5, CD1a, CD2, CD3, CD4, CD8, CD13, CD33, MPO, CD64, CD20, CD19, CD79a, and CD56. |
Go to : 
Case 2
A 14-year-old boy presented to our hospital in May 2019 with complaints of high-grade fever and epistaxis for 1-month duration. Examination revealed marked pallor and no HSM or lymphadenopathy. Complete hemogram revealed pancytopenia (hemoglobin, 5 g/dL; white cell counts, 0.8×109/L; and platelets, 20×109/L). In March 2019, BMA performed at an outside hospital was a dry tap. BM biopsy revealed extensive reticulin fibrosis (WHO grade 3) without any immature cells or granuloma. In addition, in May 2019, repeat BMA performed at our hospital was a dry tap. BM biopsy revealed reticulin fibrosis (WHO grade II) with occasional immature cells [CD34+ and CD117+ on immuno-histochemistry (IHC)]. However, immature cells could not be characterized further by IHC. The patient was closely followed up and supported with transfusions when indicated. Fever work-up was inconclusive. The vitamin-D and parathyroid hormone levels were within normal limits, and antinuclear antibodies were absent. In June 2019, PB findings were unremarkable except for occasional blasts, FCM of PB was inconclusive, and BMA was a dry tap. BM biopsy revealed dense fibrosis (WHO grade 2), dysplastic megakaryocytes, and occasional blasts. The patient was managed symptomatically with transfusion support. In August 2019, BM was successfully aspirated from the sternum. FCM of BMA revealed early T-precursor ALL (ETP-ALL, Fig. 2B). BM cytogenetic analysis revealed a normal male karyotype. He was treated with the Berlin–Frankfurt–Münster (BFM)-95 protocol for ALL. EOI BMA and biopsy on day 33 revealed morphological remission and significant resolution of BM fibrosis (WHO grade 0–1). He was continued on chemotherapy and is currently in the maintenance phase.
Go to : 
DISCUSSION
We conducted a PubMed search using the key words “acute myelofibrosis” and “acute lymphoblastic leukemia” and found 11 articles (12 cases) describing an association of MF with ALL [2-8, 12-15]. However, full-texts of only nine articles were accessible [2, 4-8, 13-15]. Four patients did not have AMF and their clinical or pathological findings were consistent with the diagnosis of PMF (two patients: tear drop cells and two patients: hepatosplenomegaly) [13-15]. Thus, only seven cases of AMF with ALL (pediatric: 4 and adult: 3) have been reported till date (Table 1) [2-8]. AMF either preceded (N=3) or occurred concurrently with the diagnosis of ALL (N=4). AMF antedated the diagnosis of ALL by 3 months to 1 year [2, 4]. HSM and lymphadenopathy were absent in all patients (N=6). Blood counts at initial presentation revealed pancytopenia (N=3), bicytopenia (N=2), and anemia (N=1). At presentation, circulating blasts (3–7%) were detected in only two patients, both of which had concurrent AMF and ALL [5, 6]. In other patients, PB blasts were detected either during subsequent follow-up (N=1) [2] or were absent (N=3) [4, 7, 8]. ALL subtyping revealed that five patients had pre-B-ALL, with two co-expressing CD20. However, ALL subtyping for two patients was unavailable. Additional BM biopsy findings included megakaryocytic hyperplasia (N=2) and megakaryocyte dysplasia (N=3). Findings of cytogenetic analysis of BM were as follows: normal (N=3), abnormal (N=2; deletion 5, and extra signal on chromosome 21q22). After treatment with ALL induction protocols, five patients were alive, of which one experienced relapse, and one patient died during therapy. Three patients were reevaluated for BM fibrosis after therapy, of which one was positive and two were negative.
Table 1
Clinical and pathological review of seven cases of acute myelofibrosis with acute lymphoblastic leukemia reported in the literature.
| S. No | Author, year | Age/sex | HSM, LN | Initial PB findings | BMA findings | ALL subtype | BM biopsy findings | Cytogenetic findings | MF at repeat BM | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Amjad et al. [2], 1979a,b) | 57 yr/F | Absent | Anaemia | Dry tap | NA | MF | Normal | NA | Death |
| 2 | Chen et al. [3], 1992a) | 4 yr/M | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 | Dunphy et al. [4], 1996a) | 44 yr/M | Absent | Pancytopenia | Dry tap | Pre-B | MF, pleomorphic and dysplastic megs | Deletion 5p | Present(EOM) | Alive |
| 4 | Abla et al.[5], 2006 | 15 yr/F | Absent | Bicytopenia,7% blasts | Diluted 9–58% blasts | Pre-B | MF (reticulin and collagen), blasts | Normal | NA | Alive |
| 5 | Avci et al. [6], 2008 | 4.5 yr/M | Absent | Bicytopenia, 3% blasts | Dry tap | Pre-B | MF | Extra signal of chr.21q22 | Absent (day-70) | Alive |
| CD20+ | ||||||||||
| 6 | Gonzalez et al. [7], 2013c) | 72 yr/M | NA | Pancytopenia | Dry tap | Pre-B | MF, clustering and dysplasia of megs, reduced megs | NA | NA | Relapse, Alive |
| CD20+ | ||||||||||
| 7 | Friesenbichler et al. [8], 2018 | 13 yr/F | Absent | Pancytopenia | Hypocellular | Pre-B | MF, megs hyperplasia and dysplasia, 15% blasts | Normal | Absent (day-33) | Alive |
| Hyperplastic and dysplastic megs, no blasts |
We reported two cases of ALL (pediatric ETP-ALL and adult Ph+-ALL). In these cases, AMF preceded the diagnosis of ALL by 5 and 3 months, respectively. No cases of ETP-ALL and Ph+-ALL presenting as AMF have been reported to date. Megakaryocyte dysplasia was present in case-2 patient. Most probably, cytokines released from lymphoblasts (megakaryocytes in case-2 patient) resulted in AMF in our patients [1, 8]. This is supported by the fact that BM fibrosis significantly reduced after ALL treatment. Secondary MF is commonly observed in CML at diagnosis [16]. Absence of HSM, eosinophilia, basophilia, dwarf megakaryocytes in the BM, and a relatively short history argued against the possibility of CML with lymphoid blast crises in case-1 patient; hence, the diagnosis of Ph+-ALL was favored. The diagnosis of ALL was delayed because the BM was inaspirable due to AMF and PB blasts were absent at presentation. Initial BM biopsies revealed only MF without any blasts. Subsequently, PB blasts were detected in both patients (case-2: 4 mo and case-1: 3 mo), which raised the suspicion of acute leukemia. Circulating blasts accounted for <20% in both patients, making PB FCM analysis difficult. Both patients required multiple transfusions during induction therapy and experienced grade-4 cytopenia. However, both patients did not have neutropenic fever and achieved morphological remission at EOI. Moreover, case-1 patient achieved molecular remission.
The current report highlights that AMF can either occur concurrently with or precede the diagnosis of acute leukemia. In the latter case, after excluding the secondary causes of MF and PMF, the suspicion of acute leukemia masquerading as AMF should be maintained. The diagnosis and subtyping of acute leukemia is frequently delayed either due to technical challenges in obtaining BM aspirate, absence of blasts in the initial BM biopsies, or low percentage of PB blasts during subsequent follow-ups, mandating close follow-up of the patients. Because dense BM fibrosis can mask the blasts, IHC analysis should be performed for immature cells in cases of AMF. Repeat BM examinations, particularly from different sites, may aid in obtaining sufficient sample for FCM analysis. ALL treatment usually results in AMF resolution. A limited number of patients preclude the evaluation of the effect of AMF on the EOI MRD and prognosis. Thus, we reported a rare presentation of ETP-ALL and Ph+-ALL and their diagnostic challenges.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download