TO THE EDITOR: The development of inhibitors [i.e., neutralizing anti-factor VIII (FVIII) alloantibodies] is a severe complication in managing hemophilia patients [1], causing significant morbidity and mortality [2]. Infusion therapy with FVIII concentrate is ineffective in sequestering high titer inhibitor (anti-FVIII ≥5 BU/mL) [3], and patients require bypassing agents (BPAs), such as activated prothrombin complex concentrates (aPCCs) and recombinant activated coagulation factor VII (rFVIIa), for prophylaxis or to stop the bleeding associated with hemophilia [3]. However, the treatment efficacy of BPAs is suboptimal, requiring frequent intravenous infusions via adequate venous access [4]. Hemophilia patients with high titer inhibitors eventually require immune tolerance induction (ITI) to eradicate inhibitors [3]. Furthermore, management with BPA or ITI leads to a high socioeconomic burden on national healthcare systems and physical and mental burden on individuals [5].
Emicizumab is a subcutaneously injected bispecific monoclonal antibody that binds to activated coagulation factor IX and X, restoring activated FVIII [4, 6]. In a phase 3 multicenter trial that included 109 patients (aged ≥12 yr) with congenital hemophilia A and high inhibitor titer, emicizumab prophylaxis (once weekly) significantly reduced the number of bleeding events compared with no prophylaxis (HAVEN 1) [4]. Similarly, in 152 hemophilia A patients without inhibitors (aged ≥12 yr), emicizumab prophylaxis significantly decreased hemorrhagic episodes compared with no prophylaxis (HAVEN 3) [7]. Moreover, emicizumab is safe and effective for treating children with hemophilia A [8, 9]. In a phase 3 clinical trial involving 85 pediatric hemophilia A patients (with inhibitors) aged <12 years (HAVEN 2), emicizumab prophylaxis showed an annual bleeding rate of only 0.3%, and 77% of participants did not require treatment for their bleeding events [9]. In another study, compared with a conventional therapy, emicizumab prophylaxis reduced the bleeding rate by 99% in 15 children previously treated with BPAs [9]. In children aged <12 years with hemophilia A (without high inhibitor titer), a multicenter, open-label study for emicizumab (HOHOEMI) revealed that emicizumab administered every 2 or 4 weeks was efficacious and safe for bleeding prophylaxis [8]. Furthermore, emicizumab has a low cost than a conventional therapy, thereby significantly reducing the burden on the families [2]. According to the Italian National Health Service, emicizumab prophylaxis saved €25.2 million and €19.98 million per patient compared with conventional therapy with rFVIIa or aPCC prophylaxis, respectively [2]. However, in Korean patients with hemophilia A, emicizumab administration is uncommon and is in its initial stage of introduction in the national health insurance.
Here, we report about a 35-month-old Korean boy with severe hemophilia A and high inhibitor titer treated with emicizumab for over 1 year. He was treated with BPAs at 6 months of age and then with emicizumab from 23 months onward. We compared the patient’s hemorrhagic episodes following conventional therapy for the past 1 year (BPAs) with episodes during 1 year of emicizumab prophylaxis. We also compared medical costs during these two treatment periods. This study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (approval No. 2019-09-063) and was conducted according to the tenets of the Declaration of Helsinki. Informed consent was obtained from the patient’s parents before the study.
To prevent intracranial hemorrhage, the patient was born via an induced labor delivery since his mother’s pelvic inlet diameter was larger than his head. His baseline FVIII was 0.1%, and bleeding events were managed with on-demand injections of recombinant FVIII concentrate. His sibling also has severe hemophilia A (baseline factor VIII 0.4%) but without inhibitors and was initially treated with recombinant FVIII concentrate. His mother is a hemophilia A carrier (baseline factor VIII 26%).
At 6 months of age, the patient’s inhibitor titer was 6.0 BU/mL, which increased to 160 BU/mL within a month after treatment with on-demand BPA. At 14 months of age, a central venous catheter (chemoport) was implanted into the patient, and ITI was scheduled to eliminate the inhibitor. However, ITI was delayed owing to severe melena that persisted for over 6 weeks. During an emergency room visit for sudden melena and pale appearance, laboratory test results revealed severe anemia (hemoglobin 30 g/L) and azotemia (BUN/Cr 31/0.33 mg/dL). He was treated with red blood cell (RBC) transfusion and BPA, but melena persisted; however, no bleeding focus was found on abdominal computed tomography, angiography, endoscopy, colonoscopy, and Meckel scan. An RBC nuclear scan revealed uneven tracer accumulation around the ileum, consistent with a bleeding focus. Since melena persisted regardless of infusion with rFVIIa and aPCC, sequential combined bypassing therapy was intensified (rFVIIa 120 mcg/kg/dose q 3 h and aPCC 70 IU/kg q 12 h) [10], and additional tranexamic acid (10 mg/kg q 6 h) was added. His melena subsided 7 weeks after hospitalization, although frequent bleeding, including hematomas and intravenous site hemorrhages, did not cease.
Because of severe bleeding tendency, we opted to treat the patient with emicizumab at its pre-launch stage in Korea. At 23 months of age, the induction dose of 3 mg/kg emicizumab was injected subcutaneously q 1 week for 4 weeks. A weekly maintenance dose of emicizumab (1.5 mg/kg) was continued for 10 weeks followed by 3 mg/kg emicizumab q 2 weeks. The medical team trained his mother on the subcutaneous administration of emicizumab. From the fifth dose (the first maintenance dose), the patient’ mother administered emicizumab. This child is now 35 months old and has received emicizumab prophylaxis for 1 year.
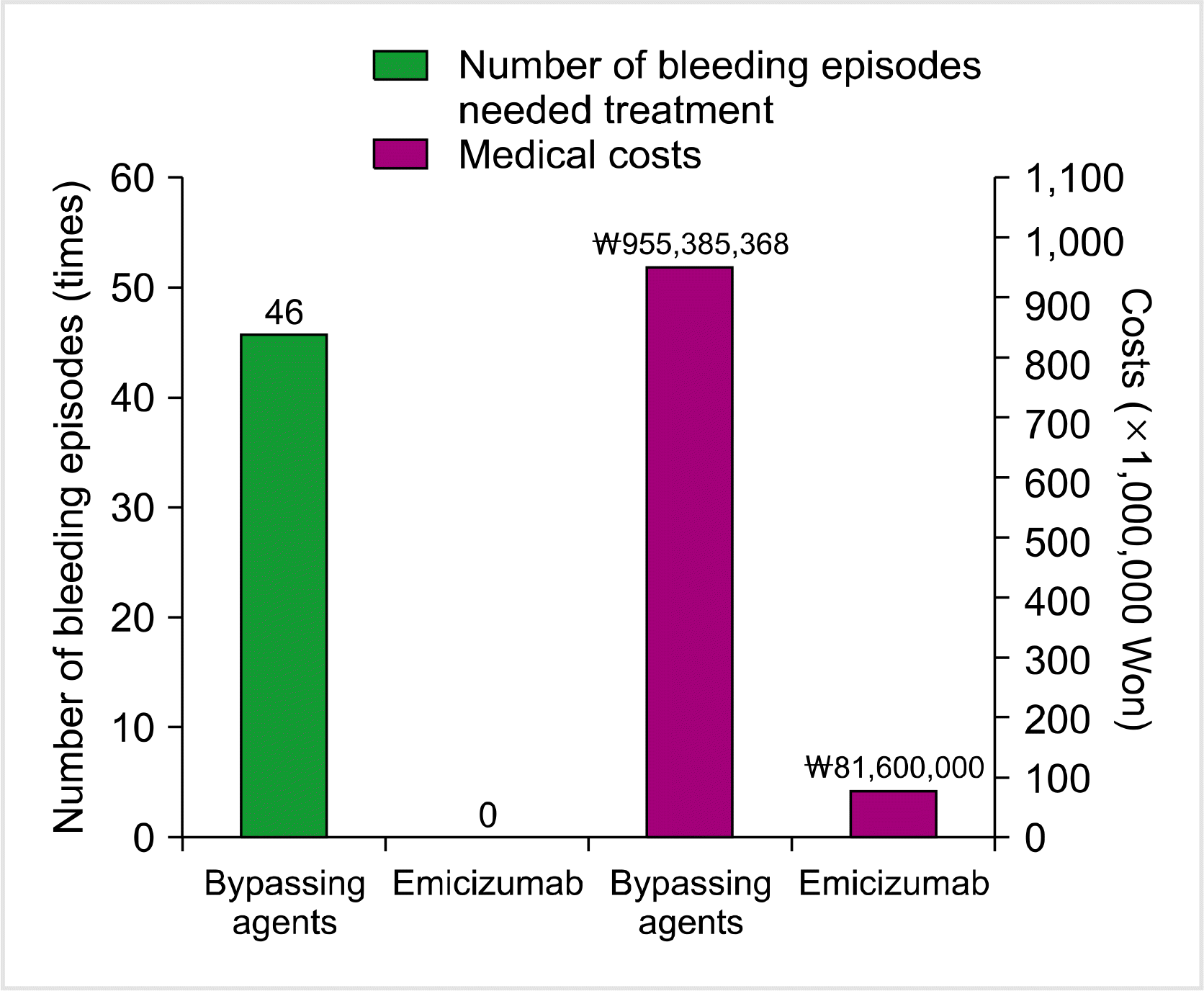
Before starting emicizumab prophylaxis, the patient required six hospital admissions and 40 visits to an outpatient clinic for hemorrhagic episodes. He had severe melena and several hematomas at his chemoport site, intravenous sites, and ankle. BPAs were injected directly into his chemoport, which was traumatic for the patient. In the case of a hematoma or infection at the chemoport site, the patient was re-admitted and received repetitive intravenous access through peripheral veins. During 1 year of emicizumab prophylaxis, the patient experienced only one episode of bruising at an injection site, which resolved spontaneously, requiring no further treatment. The patient had no thromboembolic complication or other adverse events during the study period.
The concentration of emicizumab was 69,900 ng/mL after 3 months of injection and 62,600 ng/mL after 9 months of injection (Fig. 1). FVIII inhibitor titer was 17.9 BU/mL after 3 months of emicizumab injection and 14.1 BU/mL after 9 months of emicizumab injection. After confirming the patient’s (bleeding) condition, we planned a safe chemoport removal procedure since he had experienced prolonged hospitalization owing to chemoport site hematoma or infection in the past. After 6 months of emicizumab prophylaxis (3 mg/kg every 2 wk), the chemoport catheter was removed without using other hemostatic agents. There was no hemorrhagic event during or after chemoport removal.
During 1 year of conventional treatment with BPAs before emicizumab prophylaxis, the patient required a final amount of 47,739 KIU of rFVIIa and 55,696 IU of aPCC. Applying the Korean insurance cost (₩18,440 per 1 KIU of rFVIIa and ₩1,348 per 1 IU of aPCC), the medical burden amounted to ₩955,385,368 for the treatment period. During the period (1 yr) of emicizumab prophylaxis, a total of 1,020 mg of emicizumab was consumed, and no BPA was required, costing only ₩81,600,000 at ₩80,000 per 1 mg of emicizumab, which was significantly lower than conventional treatment (<10%). The comparisons between the number of bleeding episodes and the medical costs involved for the two treatment periods (conventional therapy vs. emicizumab prophylaxis) are shown in Fig. 2.
In conclusion, prophylactic subcutaneous emicizumab injection every 1–2 weeks was effective and safe for treating the Korean boy with severe hemophilia A and a high titer inhibitor. Emicizumab dramatically reduced bleeding episodes, the use of BPA, and overall medical burden. The patient had a history of complications, including severe melena, chemoport site hematoma, and large hematomas at other body sites during conventional treatment; conversely, this patient had no bleeding episodes requiring BPAs during emicizumab prophylaxis. Additionally, an invasive procedure such as chemoport removal operation was possible for the patient during emicizumab prophylaxis, without any adverse events. Emicizumab treatment resulted in improved quality of life and convenience for the patient and his parents.
REFERENCES
1. Walsh CE, Soucie JM, Miller CH. United States Hemophilia Treatment Center Network. 2015; Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 90:400–5. DOI: 10.1002/ajh.23957. PMID: 25616111.

2. Cortesi PA, Castaman G, Trifirò G, et al. 2020; Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia a patients with inhibitors. Thromb Haemost. 120:216–28. DOI: 10.1055/s-0039-3401822. PMID: 31887777.

3. Witmer C, Young G. 2013; Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol. 4:59–72. DOI: 10.1177/2040620712464509. PMID: 23610614. PMCID: PMC3629762.


4. Oldenburg J, Mahlangu JN, Kim B, et al. 2017; Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 377:809–18. DOI: 10.1056/NEJMoa1703068. PMID: 28691557.

5. D'Angiolella LS, Cortesi PA, Rocino A, et al. 2018; The socioeconomic burden of patients affected by hemophilia with inhibitors. Eur J Haematol. 101:435–56. DOI: 10.1111/ejh.13108. PMID: 29889317.

6. Kitazawa T, Igawa T, Sampei Z, et al. 2012; A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 18:1570–4. DOI: 10.1038/nm.2942. PMID: 23023498.

7. Mahlangu J, Oldenburg J, Paz-Priel I, et al. 2018; Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 379:811–22. DOI: 10.1056/NEJMoa1803550. PMID: 30157389.

8. Shima M, Nogami K, Nagami S, et al. 2019; A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 25:979–87. DOI: 10.1111/hae.13848. PMID: 31515851. PMCID: PMC6900083.


9. Young G, Liesner R, Chang T, et al. 2019; A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 134:2127–38. DOI: 10.1182/blood.2019001869. PMID: 31697801. PMCID: PMC6908828.
10. Gringeri A, Fischer K, Karafoulidou A, et al. 2011; Sequential combined bypassing therapy is safe and effective in the treatment of unresponsive bleeding in adults and children with haemophilia and inhibitors. Haemophilia. 17:630–5. DOI: 10.1111/j.1365-2516.2010.02467.x. PMID: 21323801.

Fig. 1
Concentration of emicizumab during prophylaxis in a Korean pediatric patient with severe hemophilia A and high titer inhibitor.
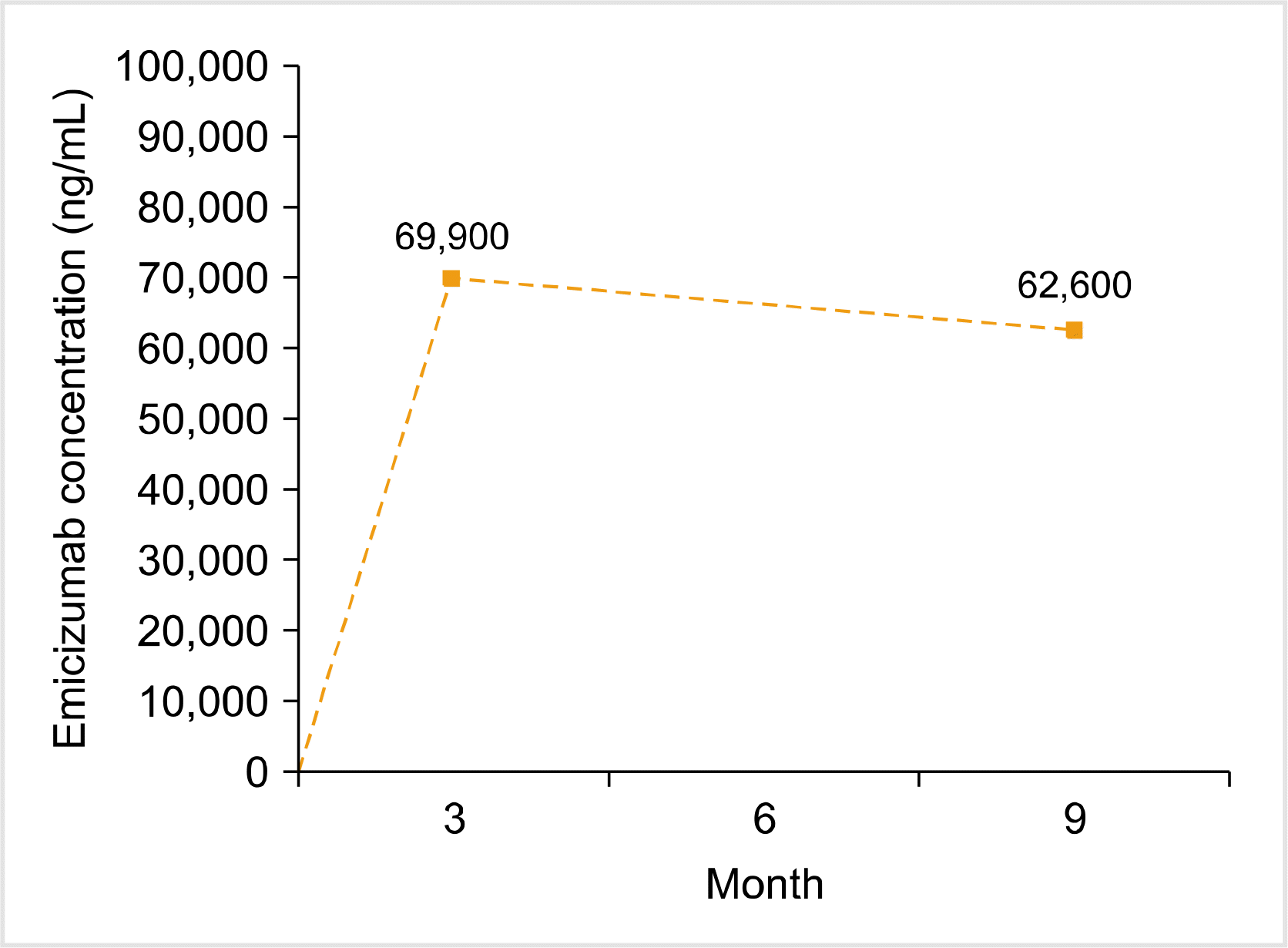
Fig. 2
A comparison of the number of bleeding episodes that needed treatment and of the medical costs between the previous 1 year (conventional therapy using bypassing agents) and the present 1 year (emicizumab prophylaxis) in a Korean pediatric patient with severe hemophilia A and high titer inhibitor.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download