Abstract
The association between the risk of allograft rejection after organ transplantation and FAS gene polymorphism has been evaluated previously. However, inconsistent results have been reported. Hence, we conducted the most up-to-date meta-analysis to evaluate this association. All eligible studies reporting the association between FAS-670A>G polymorphism and the risk of allograft rejection published up to December 2019 were extracted using a comprehensive systematic database search in the Web of Science, Scopus, and PubMed. The pooled odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated to determine the association strength. This meta-analysis included six case-control studies with 277 patients who experienced allograft rejection and 1,001 patients who did not experience allograft rejection (controls) after organ transplantation. The overall results showed no significant association between FAS-670A>G polymorphism and the risk of allograft rejection in five genetic models (dominant model: OR=0.81, 95% CI=0.58‒1.12; recessive model: OR=0.10, 95% CI=0.80‒1.53; allelic model: OR=0.96, 95% CI=0.79‒1.18; GG vs. AA: OR=0.92, 95% CI=0.62‒1.36; and AG vs. AA: OR=0.75, 95% CI=0.52‒1.08). Moreover, subgroup analysis according to ethnicity and age did not reveal statistically significant results. Our findings suggest that FAS-670A>G polymorphism is not associated with the risk of allograft rejection after organ transplantation.
Organ transplantation, such as renal, liver, and heart transplantation, is the best therapeutic option for most patients with end-stage disease [1]. Over the past decades, due to new advances in surgical techniques, expansion of effective immunosuppressive agents, and better recognition of alloimmune response and histocompatibility matching, the short- and long-term graft survival outcomes in transplant recipients have improved [2]. However, immunosuppressive protocols have increased the rates of infection and malignancy in patients undergoing organ transplantation [3]. Therefore, it is important to identify the factors that influence the risk of rejection in such diseases. A growing body of evidence supports that apoptosis contributes to graft rejection and the establishment of tolerance in transplantation.
FAS is one of the most important inducers of the apoptotic pathway [4]. FAS (also known as CD95/TNFSF6/APO-1) is a cell surface receptor belonging to the tumor necrosis factor receptor (TNF-R) family and is highly expressed in a wide range of cells, including lymphocytes, neutrophils, monocytes, and tissues such as the heart, kidney, and liver [5, 6]. Its gene, located on chromosome 10q24.1, consists of nine exons and eight introns and is highly polymorphic [7]. Apoptosis plays a pivotal role in the deletion of self-reactive lymphocytes, including immature T cells and peripheral mature T cells [8], and death of target cells by effector cytotoxic T lymphocytes (CTLs) [9]. Significant depletion of renal tubular epithelial cells by apoptosis in kidney recipients experiencing acute or chronic allograft rejection has been described [10]. Moreover, hepatocyte apoptosis has been detected in acute liver graft rejection [11]. However, some studies have shown that apoptosis of activated T cells within accepted grafts plays a significant role in inducing hepatic tolerance [12].
Some studies have suggested that the FAS gene is controlled by various genetic elements positioned in the 5-upstream promoter regions of the gene, especially in the transcription factor binding sites [13]. However, a functional polymorphism involving an A→G transition at position ‑670 in the enhancer region (Fas-670A>G, rs1800682) of FAS has been reported. This polymorphism destroys signal transducer activator of transcription 1 (STAT1), consequently reducing promoter activity and diminishing FAS expression [14, 15]. Because of the importance of this single nucleotide polymorphism in the susceptibility of recipient T cells to FASL-mediated apoptosis [16], we performed a meta-analysis to determine whether Fas-670A>G gene polymorphism is associated with the risk of allograft rejection after organ transplantation.
The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [17], including search strategy, inclusion and exclusion criteria, data extraction and quality assessment, and statistical analysis.
All studies reporting the association between FAS-670A>G polymorphism and susceptibility to allograft rejection after organ transplantation until December 2019 were retrieved by a systematic search of PubMed, Scopus, and Web of Science. The following combinations of keywords were used: (“rejection” or “graft failure”) and (“APO-1” or “TNFSF6” or “CD95” or “FAS”) and (“polymorphism” or “variation” or “mutation” or “allele” or “genotype” or “SNP” or “single nucleotide polymorphism”). Furthermore, we manually screened the reference lists of eligible studies and relevant reviews to identify missing data during the electronic search.
Studies were considered eligible if they met the following criteria: a) studies that evaluated the association between allograft rejection and FAS-670A>G polymorphism; b) studies providing adequate data to calculate the odds ratio (OR) and its 95% confidence interval (CI), and c) studies including two comparison groups (rejection group vs. non-rejection group). Other studies, such as review articles, book chapters, editorials, comments, abstracts, duplicated data, and republished articles, were excluded.
Two authors independently extracted the following data according to an extraction checklist: first author’s name, journal and year of publication, ethnicity, country of origin, mean of age, methods for genotyping, sample size of cases and controls, and the number of cases and controls for each genotype. Any discrepancies between the two reviewers were discussed and resolved by consensus. The quality of each study was assessed using the Newcastle-Ottawa Scale (NOS) criteria [18]. Studies with scores of 0–3, 4–6, or 7–9 were considered low-, moderate-, or high-quality studies, respectively.
For each case-control study, deviation from the Hardy-Weinberg equilibrium was analyzed using the c2 test in the control group. The pooled OR and 95% CI were computed to evaluate the strength of associations between FAS-670A>G gene polymorphism and the risk of rejection after organ transplantation. Different possible comparison models for FAS-670A>G gene single-nucleotide polymorphism (SNP) included the dominant model (GG+AG vs. AA), recessive model (GG vs. AG+AA), allelic model (G vs. A), homozygote (GG vs. AA), and heterozygote (AG vs. AA). Heterogeneity among the included studies was measured using Q statistics (P<0.1 was considered statistically significant) and I2 test (I2 values of 25%, 50%, and 75% were described as low, moderate, and high heterogeneity, respectively) [19, 20]. If heterogeneity was detected, a random effects model (Der Simonian-Laird approach) was used; otherwise, the fixed effects model (Mantel-Haenszel approach) was used (Q statistic P>0.1 or I2<50%) [21]. Sensitivity analysis was used to evaluate the stability of our results. Publication bias was estimated using funnel plots and Begg’s and Egger’s tests [22, 23] (P<0.05 was considered statistically significant). This meta-analysis was performed using STATA 14.0 software (State Corporation, College Station, TX, USA).
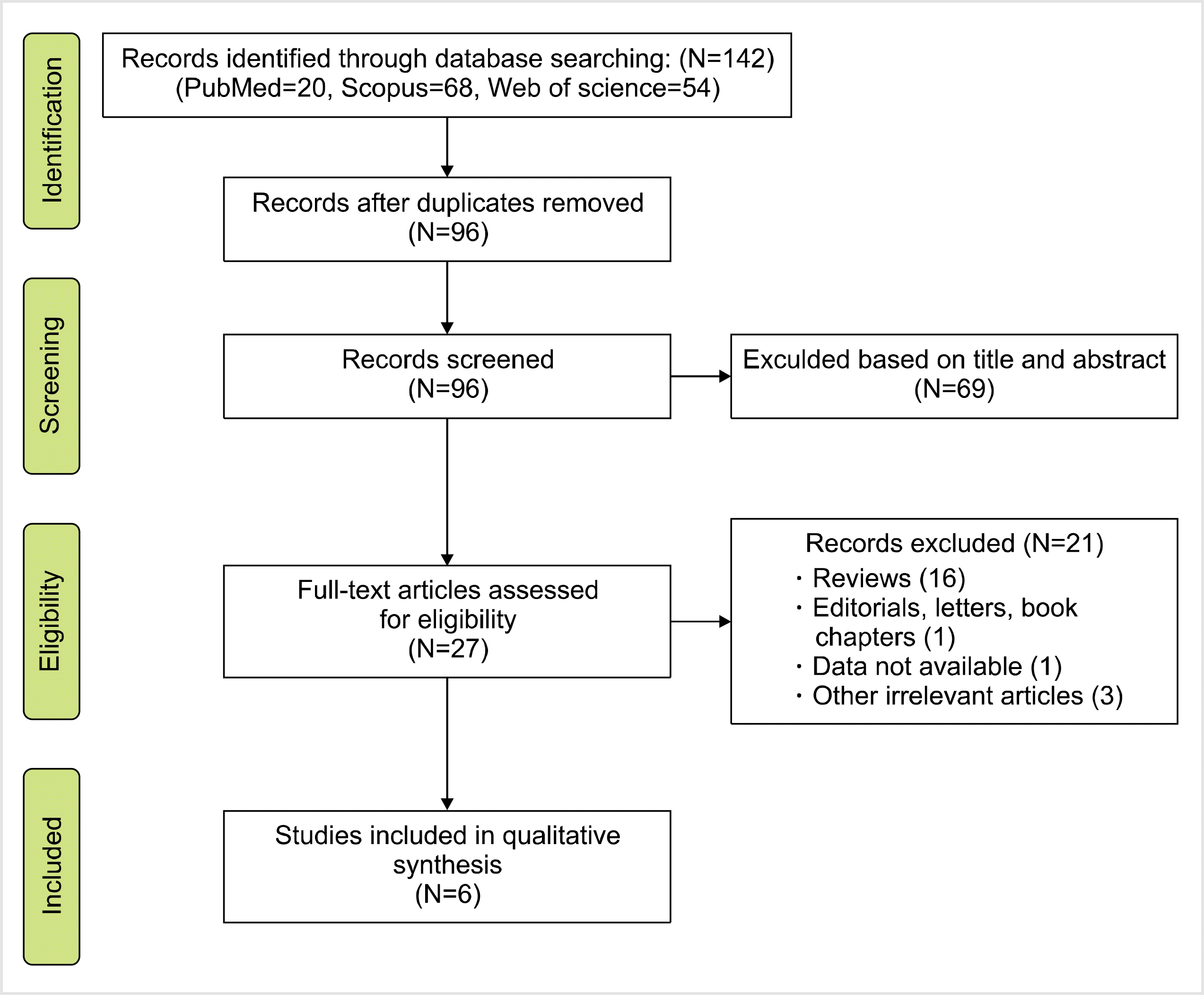
Fig. 1 shows the flow diagram of the study selection process according to the PRISMA guidelines. In total, 142 studies were identified during the primary search. Subsequently, duplicates (N=46) were omitted, and other studies were excluded either by title and abstract (N=69) or full-text (N=21) screening. Eventually, six publications reporting the association between FAS-670A>G gene polymorphism and the risk of rejection were included in the quantitative analysis [16, 24-28]. The studies were performed in different countries, including Iran, France, Spain, Turkey, and Egypt. All eligible studies had good overall methodological scores, ranging from 6 to 8. Restriction fragment length polymorphism was the common genotyping method used in the included studies. The characteristics, allele frequency, and genotype distributions of the included studies are summarized in Tables 1 and 2.
We analyzed all eligible studies on the association between FAS-670A>G polymorphism and the risk of allograft rejection after organ transplantation. The pooled effect size indicated that there was no significant association between FAS-670A>G gene polymorphisms and the risk of allograft rejection across the different genotype models—dominant model (OR=0.81, 95% CI=0.58–1.12, P=0.19, REM), recessive model (OR=0.10, 95% CI=0.80–1.53, P=0.55, REM), allelic model (OR=0.96, 95% CI=0.79, 1.18, P=0.7, REM), GG vs. AA model (OR=0.92, 95% CI=0.62–1.36, P=0.66, REM), and AG vs. AA model (OR=0.75, 95% CI=0.52–1.08, P=0.12, REM) (Figs. 2–6).
We categorized studies according to ethnicity—Caucasians (4 articles), mixed (1 article), and Arabs (1 article). Since there was only one study for the mixed and Arab populations, these studies were excluded from the analysis. The results of subgroup analysis in the Caucasian population did not reveal any significant association between FAS-670A>G gene polymorphisms and the risk of allograft rejection in all genetic models. Additionally, categorized studies according to age—children (3 articles) and adults (2 articles). The results did not reveal any statistically significant association. The details are listed in Table 3.
No significant heterogeneity was identified in the meta-analysis. Additionally, Egger’s linear regression and Begg’s funnel plot test were used to evaluate publication bias. The shape of the funnel plot did not reveal obvious asymmetry in any of the genotype models of FAS-670A>G gene polymorphism (Table 3).
The impact of individual studies on the pooled OR was evaluated by sequential omission of each study. The results showed that no individual study significantly affected the pooled OR in all genotype models of FAS-670A>G polymorphism (Fig. 7).
To date, several individual case-control replication studies have attempted to investigate the association between the FAS-670A>G gene polymorphism and the risk of allograft rejection after organ transplantation. Due to some differences, however, these dispersed investigations have demonstrated incongruous reports. However, a meta-analysis is a tool that has the potential to solve the problem of inconsistency by removing the confining issues of insufficient statistical power in individual studies. Therefore, to resolve the mentioned confining factors of the FAS-670A>G gene polymorphism, the most recent meta-analysis was conducted to determine a bona fide estimation of the association between the FAS-670A>G gene polymorphism and allograft rejection after organ transplantation. Our findings indicated that FAS-670A>G gene polymorphism was not associated with the risk of allograft rejection after organ transplantation in the overall population. In addition, subgroup analysis according to ethnicity and age showed no significant association between FAS-670A>G gene polymorphism and the risk of allograft rejection.
Programmed cell death (apoptosis) is an essential physiological mechanism involved in the development and homeostasis of the immune system. The main mechanism of apoptosis is the extrinsic pathway involving surface molecules known as “death receptors” and their ligands, the best-characterized death receptor including FAS [29]. Engagement of the T-cell receptor/CD3 complex upregulates CD95 expression and induces CD95L expression through antigen stimulation. Through these cell surface molecules, activated T cells undergo activation-induced cell death, which is principally mediated by the CD95/CD95L system to develop spontaneous tolerance to the allograft [30, 31]. Consistent with these theoretical points, Boix et al. [32] and Mancebo et al. [33] reported that patients who experienced liver and kidney rejection, respectively, had higher levels of CD95 in both CD4 and CD8 T cells within the first month after transplantation. In addition, Wang et al. [34] observed that CD95 expression on CD3+ T cells in liver transplantation rejection compared to that in stable recipients or healthy individuals was significantly increased. Therefore, in patients experiencing allograft rejection, we cannot deny the role of allograft infiltrated T cells with overexpressed CD95 that induces apoptosis through the establishment of the CD95-CD95L complex and stimulates the rejection process.
FAS-670A>G and FAS-1377G/A are two important SNPs that have been reported in the FAS promoter region [14]. The first one with the G variant disrupts the interferon-gamma binding site for the transcription factor STAT1. FAS was significantly upregulated by interferon-gamma in several reports [35-37]. Therefore, healthy subjects who are homozygous for the 670 A/A major allele have higher levels of FAS expression than those who are homozygous for the 670 G/G variant [14], which in turn could decrease their capability to be depleted by apoptosis. Moreover, FAS and FASL may occur as cell surface proteins or in soluble forms [38]. Various isoforms of soluble FAS (sFAS) are generated by alternative splicing of FAS, and the most frequent sFAS isoform results from the deletion of exon 6, which encodes the last five amino acids of the extracellular domain and 16 of the 17 amino acids of the transmembrane domain, which is thought to prevent the function of FAS [39, 40].
According to this mechanism, FAS-670A>G gene polymorphism probably influences the risk of organ rejection. The FAS/FASL system plays a significant role in progressive renal disease and organ rejection in liver [41], cardiac [39], and renal transplantation [42]. For example, liver transplant recipients carrying the FAS-670AA genotype displayed significantly lower graft survival rates than those carrying the AG genotype [25]. In addition, low levels of soluble FAS are present in the serum of normal individuals, and enhanced serum concentrations of sFAS have been reported in bone marrow transplantation [43], chronic kidney allograft rejection [44], and acute liver allograft rejection [45]. Wang et al. [46] reported that overexpression of sFAS in allograft endothelium reduced vascular cell apoptosis, infiltration of the arterial wall by leukocytes, and disruption of the media layer in a rat aortic allograft model of chronic rejection. Further investigation revealed that FAS-670A>G gene polymorphism could regulate sFAS expression, and normal patients carrying the FAS-A/A genotype produced markedly higher levels of sFAS than those carrying the sFAS-G/G genotype [47].
This meta-analysis has some limitations and limitations. First, the analysis was based on a crude estimation of FAS-670A>G gene polymorphism association with allograft rejection, regardless of the effect of confounding factors such as age, sex, environmental factors, and the contribution of other genes in LD with the FAS gene. Second, because of the limited number of studies on other SNPs, we did not analyze other SNPs of the FAS gene that could contribute to the understanding of FAS SNP involvement in allograft rejection. Third, we were unable to perform subgroup analyses according to sex and clinical or environmental variables. Fourth, although we used a comprehensive search strategy, the number of eligible studies for quantitative analysis was low, and we strongly suggest that our findings should be interpreted with caution. Fifth, since there was no meta-analysis on FAS gene polymorphism and its association with age and ethnicity, we could not compare our findings.
In conclusion, the present meta-analysis demonstrated that there was no significant independent association between Fas-670A>G gene polymorphism and the risk of allograft rejection after organ transplantation. To reach a definitive conclusion, more well-designed studies with larger samples are necessary to clarify the role of this polymorphism in allograft rejection risk.
ACKNOWLEDGMENTS
We thank Dr. Bahman Razi for his valuable comments that greatly improved the manuscript. ME and RR originated the study and acquired data. ME and MA performed the statistical analysis, interpreted the data, and drafted the manuscript. AD and revised the manuscript. All authors have read and approved the final manuscript.
REFERENCES
1. Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. 2018; Solid organ transplantation in the 21 st century. Ann Transl Med. 6:409. DOI: 10.21037/atm.2018.09.68. PMID: 30498736. PMCID: PMC6230860.


2. Ruiz P, Maldonado P, Hidalgo Y, et al. 2013; Transplant tolerance: new insights and strategies for long-term allograft acceptance. Clin Dev Immunol. 2013:210506. DOI: 10.1155/2013/210506. PMID: 23762087. PMCID: PMC3665173.

3. Benvenuto LJ, Anderson MR, Arcasoy SM. 2018; New frontiers in immunosuppression. J Thorac Dis. 10:3141–55. DOI: 10.21037/jtd.2018.04.79. PMID: 29997983. PMCID: PMC6006112.



4. Zavazava N, Kabelitz D. 2000; Alloreactivity and apoptosis in graft rejection and transplantation tolerance. J Leukoc Biol. 68:167–74. PMID: 10947059.

5. Nwakoby IE, Reddy K, Patel P, et al. 2001; Fas-mediated apoptosis of neutrophils in sera of patients with infection. Infect Immun. 69:3343–9. DOI: 10.1128/IAI.69.5.3343-3349.2001. PMID: 11292757. PMCID: PMC98293.



6. Martinez OM, Krams SM. 1999; Involvement of Fas-Fas ligand interactions in graft rejection. Int Rev Immunol. 18:527–46. DOI: 10.3109/08830189909088497. PMID: 10672500.


7. Singh R, Pradhan V, Patwardhan M, Ghosh K. 2009; APO-1/Fas gene: structural and functional characteristics in systemic lupus erythematosus and other autoimmune diseases. Indian J Hum Genet. 15:98–102. DOI: 10.4103/0971-6866.60184. PMID: 21088713. PMCID: PMC2922636.



8. Xing Y, Hogquist KA. 2012; T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 4:a006957. DOI: 10.1101/cshperspect.a006957. PMID: 22661634. PMCID: PMC3367546.

9. Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R. 2009; Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 6:15–25. DOI: 10.1038/cmi.2009.3. PMID: 19254476. PMCID: PMC4002546.



10. Priante G, Gianesello L, Ceol M, Del Prete D, Anglani F. 2019; Cell death in the kidney. Int J Mol Sci. 20:3598. DOI: 10.3390/ijms20143598. PMID: 31340541. PMCID: PMC6679187.


11. Tannapfel A, Kohlhaw K, Ebelt J, et al. 1999; Apoptosis and the expression of Fas and Fas ligand (FasL) antigen in rejection and reinfection in liver allograft specimens. Transplantation. 67:1079–83. DOI: 10.1097/00007890-199904150-00027. PMID: 10221500.

12. Crispe IN. 2003; Hepatic T cells and liver tolerance. Nat Rev Immunol. 3:51–62. DOI: 10.1038/nri981. PMID: 12511875.


13. Nunobiki O, Ueda M, Toji E, et al. 2011; Genetic polymorphism of cancer susceptibility genes and HPV infection in cervical carcinogenesis. Patholog Res Int. 2011:364069. DOI: 10.4061/2011/364069. PMID: 21660264. PMCID: PMC3108378.

14. Huang QR, Morris D, Manolios N. 1997; Identification and character-ization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 34:577–82. DOI: 10.1016/S0161-5890(97)00081-3. PMID: 9393960.

15. Sibley K, Rollinson S, Allan JM, et al. 2003; Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 63:4327–30. PMID: 12907599.

16. Ertan P, Mir S, Ozkayin N, Berdeli A. 2010; Association of FAS -670A/G and FASL -843C/T gene polymorphisms on allograft nephropathy in pediatric renal transplant patients. Iran J Pediatr. 20:442–50. PMID: 23056744. PMCID: PMC3446084.


17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. 2010; Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 8:336–41. DOI: 10.1016/j.ijsu.2010.02.007. PMID: 20171303.


18. Stang A. 2010; Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25:603–5. DOI: 10.1007/s10654-010-9491-z. PMID: 20652370.


19. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. 2006; Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 11:193–206. DOI: 10.1037/1082-989X.11.2.193. PMID: 16784338.

20. DerSimonian R, Laird N. 1986; Meta-analysis in clinical trials. Control Clin Trials. 7:177–88. DOI: 10.1016/0197-2456(86)90046-2. PMID: 3802833.


21. Mantel N, Haenszel W. 1959; Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 22:719–48. PMID: 13655060.

22. Begg CB, Mazumdar M. 1994; Operating characteristics of a rank correlation test for publication bias. Biometrics. 50:1088–101. DOI: 10.2307/2533446. PMID: 7786990.


23. Egger M, Davey Smith G, Schneider M, Minder C. 1997; Bias in meta-analysis detected by a simple, graphical test. BMJ. 315:629–34. DOI: 10.1136/bmj.315.7109.629. PMID: 9310563. PMCID: PMC2127453.



24. Cappellesso S, Valentin JF, Al-Najjar A, et al. 2002; Recipient TNFRSF6 (FAS) gene polymorphism and acute renal allograft rejection. Transplant Proc. 34:803–4. DOI: 10.1016/S0041-1345(01)02916-5. PMID: 12034188.


25. Marín LA, Muro M, Moya-Quiles MR, et al. 2006; Study of Fas (CD95) and FasL (CD178) polymorphisms in liver transplant recipients. Tissue Antigens. 67:117–26. DOI: 10.1111/j.1399-0039.2006.00538.x. PMID: 16441482.


26. Jahadi Hosseini HR, Kamali Sarvestani E, Akbari M, Mosallaei M. 2009; Effect of Fas-670 A/G gene polymorphism on corneal allograft endothelial rejection. Iran J Immunol. 6:28–32. PMID: 19293475.

27. Girnita DM, Ohmann EL, Brooks MM, et al. 2011; Gene polymorphisms impact the risk of rejection with hemodynamic compromise: a multicenter study. Transplantation. 91:1326–32. DOI: 10.1097/TP.0b013e31821c1e10. PMID: 21659963.


28. Fadel FI, Elshamaa MF, Salah A, et al. 2016; Fas/Fas Ligand pathways gene polymorphisms in pediatric renal allograft rejection. Transpl Immunol. 37:28–34. DOI: 10.1016/j.trim.2016.04.006. PMID: 27109035.


29. Elmore S. 2007; Apoptosis: a review of programmed cell death. Toxicol Pathol. 35:495–516. DOI: 10.1080/01926230701320337. PMID: 17562483. PMCID: PMC2117903.



30. Carroll HP, Ali S, Kirby JA. 2001; Accelerating the induction of Fas-mediated T cell apoptosis: a strategy for transplant tolerance? Clin Exp Immunol. 126:589–97. DOI: 10.1046/j.1365-2249.2001.01706.x. PMID: 11737081. PMCID: PMC1906223.



31. Klemke CD, Brenner D, Weiss EM, et al. 2009; Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res. 69:4175–83. DOI: 10.1158/0008-5472.CAN-08-4631. PMID: 19435902.


32. Boix F, Millan O, San Segundo D, et al. 2016; High expression of CD38, CD69, CD95 and CD154 biomarkers in cultured peripheral T lymphocytes correlates with an increased risk of acute rejection in liver allograft recipients. Immunobiology. 221:595–603. DOI: 10.1016/j.imbio.2016.01.008. PMID: 26850323.


33. Mancebo E, Castro MJ, Allende LM, et al. 2016; High proportion of CD95(+) and CD38(+) in cultured CD8(+) T cells predicts acute rejection and infection, respectively, in kidney recipients. Transpl Immunol. 34:33–41. DOI: 10.1016/j.trim.2016.01.001. PMID: 26773856.


34. Wang YL, Zhang YY, Li G, et al. 2005; Correlation of CD95 and soluble CD95 expression with acute rejection status of liver trans-plantation. World J Gastroenterol. 11:1700–4. DOI: 10.3748/wjg.v11.i11.1700. PMID: 15786554. PMCID: PMC4305958.

35. Kanemitsu S, Ihara K, Saifddin A, et al. 2002; A functional polymo-rphism in fas (CD95/APO-1) gene promoter associated with systemic lupus erythematosus. J Rheumatol. 29:1183–8. PMID: 12064832.

36. Farre L, Bittencourt AL, Silva-Santos G, et al. 2008; Fas 670 promoter polymorphism is associated to susceptibility, clinical pre-sentation, and survival in adult T cell leukemia. J Leukoc Biol. 83:220–2. DOI: 10.1189/jlb.0407198. PMID: 17962369.

37. Razi B, Alizadeh S, Imani D, Rezaei R, Omidkhoda A. 2017; Interferon-gamma +874 (T/A) polymorphism and susceptibility to aplastic anemia: a systematic review and meta-analysis. Evid Based Med Pract. 3:1000112. DOI: 10.4172/2471-9919.1000112.

38. Ding YW, Pan SY, Xie W, Shen HY, Wang HH. 2018; Elevated soluble Fas and FasL in cerebrospinal fluid and serum of patients with anti-N-methyl-D-aspartate receptor encephalitis. Front Neurol. 9:904. DOI: 10.3389/fneur.2018.00904. PMID: 30410466. PMCID: PMC6209679.



39. Pérez EC, Shulzhenko N, Morgun A, et al. 2006; Expression of Fas, FasL, and soluble Fas mRNA in endomyocardial biopsies of human cardiac allografts. Hum Immunol. 67:22–6. DOI: 10.1016/j.humimm.2006.02.037. PMID: 16698421.


40. Papoff G, Cascino I, Eramo A, Starace G, Lynch DH, Ruberti G. 1996; An N-terminal domain shared by Fas/Apo-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 156:4622–30. PMID: 8648105.

41. Adachi K, Fujino M, Kitazawa Y, et al. 2006; Exogenous expression of Fas-ligand or CrmA prolongs the survival in rat liver transplantation. Transplant Proc. 38:2710–3. DOI: 10.1016/j.transproceed.2006.08.011. PMID: 17098047.


42. Ortiz A. 2000; Nephrology forum: apoptotic regulatory proteins in renal injury. Kidney Int. 58:467–85. DOI: 10.1046/j.1523-1755.2000.00188.x. PMID: 10886604.

43. Liem LM, van Lopik T, van Nieuwenhuijze AE, van Houwelingen HC, Aarden L, Goulmy E. 1998; Soluble Fas levels in sera of bone marrow transplantation recipients are increased during acute graft-versus-host disease but not during infections. Blood. 91:1464–8. DOI: 10.1182/blood.V91.4.1464. PMID: 9454779.


44. Nishioka T, Minami T, Matsumoto S, et al. 2000; Soluble FAS in renal allograft recipients. Transplant Proc. 32:1784. DOI: 10.1016/S0041-1345(00)01375-0. PMID: 11119935.


45. Rivero M, Crespo J, Mayorga M, Fábrega E, Casafont F, Pons-Romero F. 2002; Involvement of the Fas system in liver allograft rejection. Am J Gastroenterol. 97:1501–6. DOI: 10.1111/j.1572-0241.2002.05797.x. PMID: 12094873.


46. Wang T, Dong C, Stevenson SC, et al. 2002; Overexpression of soluble FAS attenuates transplant arteriosclerosis in rat aortic allografts. Circulation. 106:1536–42. DOI: 10.1161/01.CIR.0000027822.23269.07. PMID: 12234961.


47. Mahfoudh W, Bel Hadj ad B Jr, Romdhane A, Chouchane L. 2007; A polymorphism in FAS gene promoter correlated with circulating soluble FAS levels. Int J Immunogenet. 34:209–12. DOI: 10.1111/j.1744-313X.2007.00676.x. PMID: 17504511.


Fig. 2
Forest plot of the association between FAS-670A>G gene single-nucleotide polymorphism and the risk of allograft rejection in the dominant model.
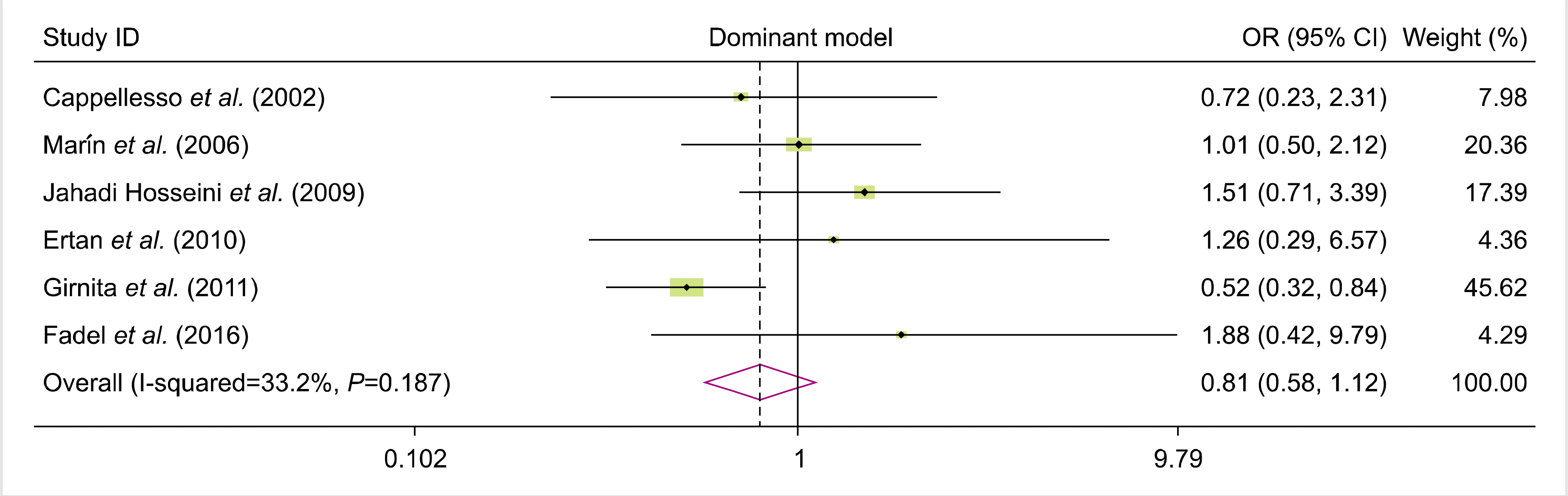
Fig. 3
Forest plot of the association between FAS-670A>G gene single-nucleotide polymorphism and the risk of allograft rejection in the recessive model.
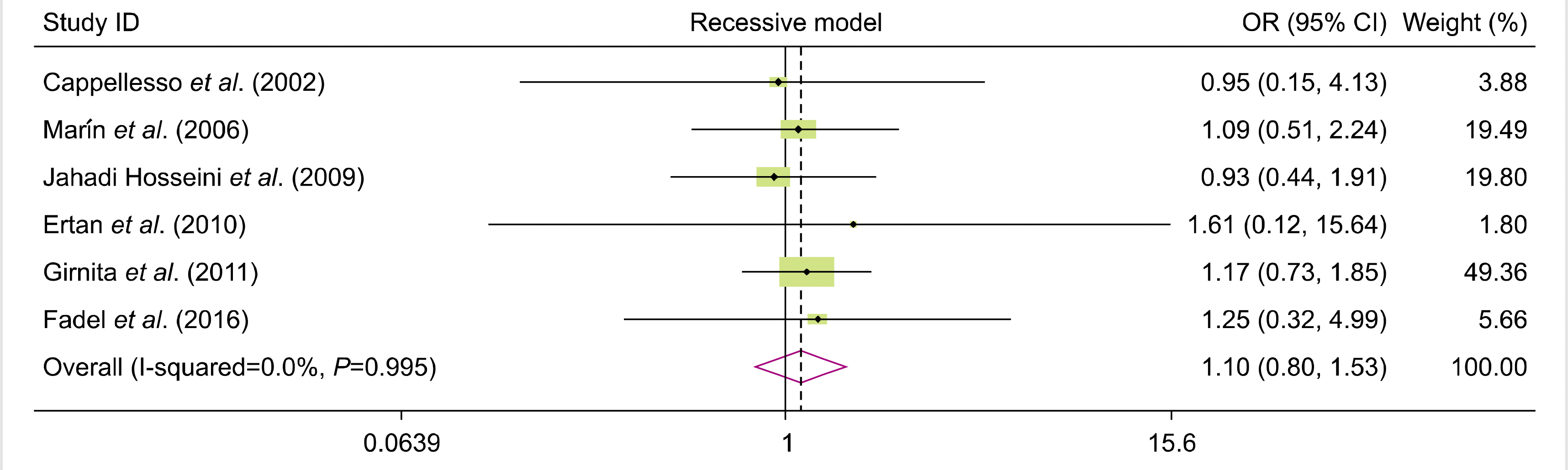
Fig. 4
Forest plot of the association between FAS-670A>G gene single-nucleotide polymorphism and the risk of allograft rejection in the allelic model.
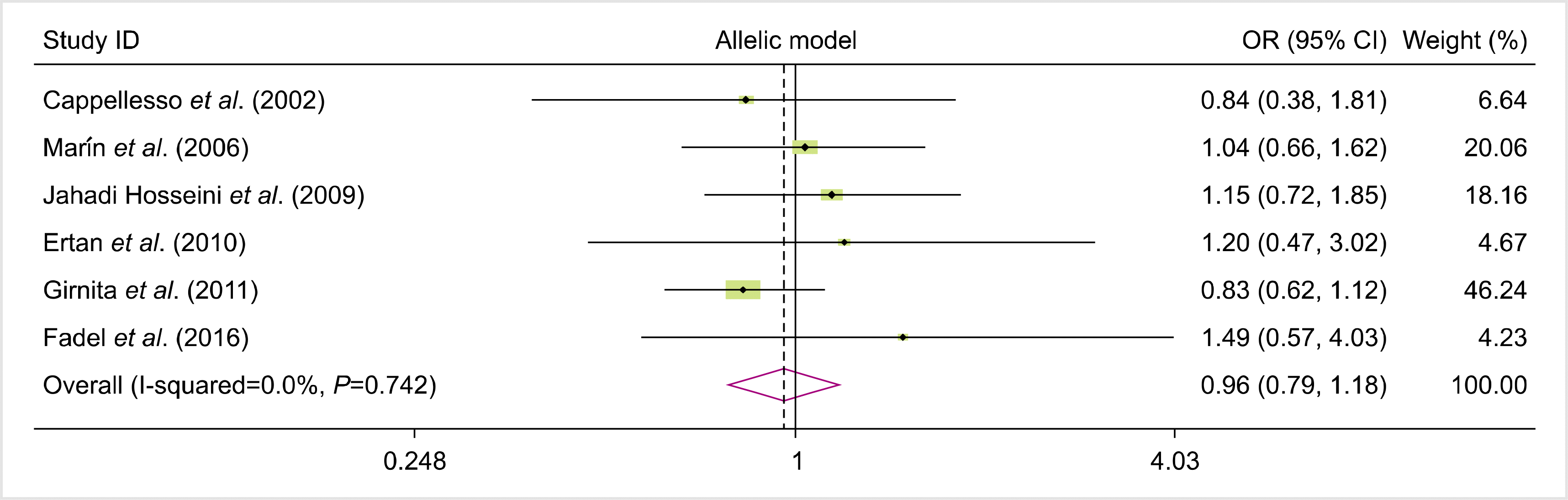
Fig. 5
Forest plot of the association between FAS-670A>G gene single-nucleotide polymorphism and the risk of allograft rejection in the AG vs. AA model.
Fig. 6
Forest plot of the association between FAS-670A>G gene single-nucleotide polymorphism and the risk of allograft rejection in the GG vs. AA model.
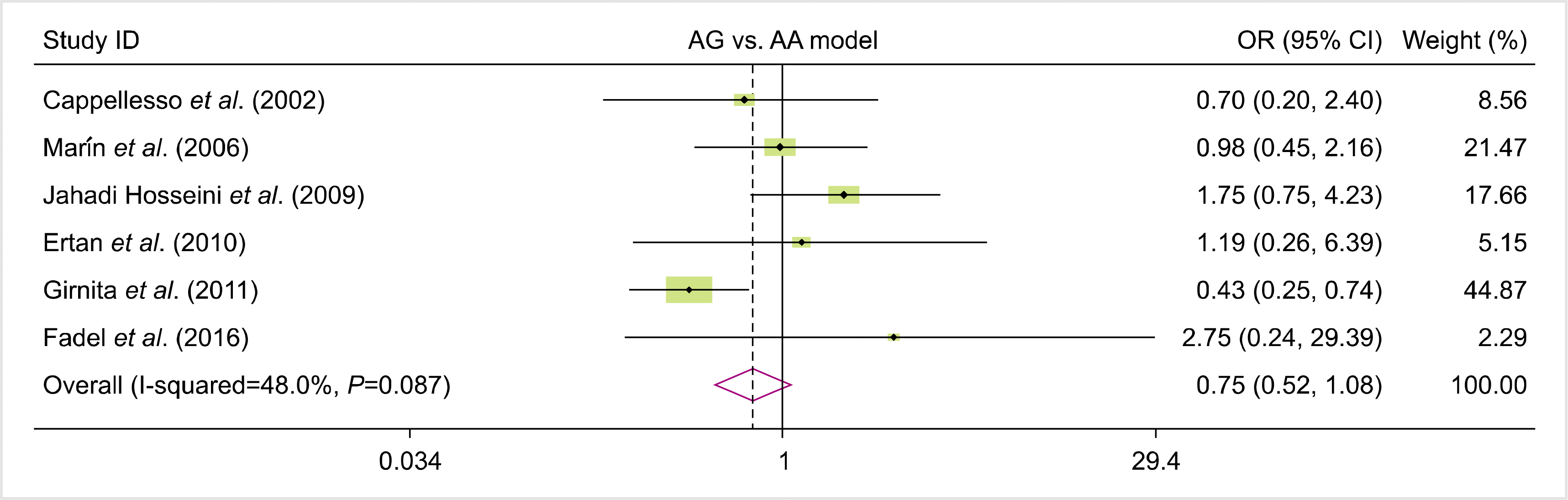
Fig. 7
Sensitivity analysis to investigate whether FAS-670A/G gene single nucleotide polymorphism contributes to risk for allograft rejection (Recessive model).
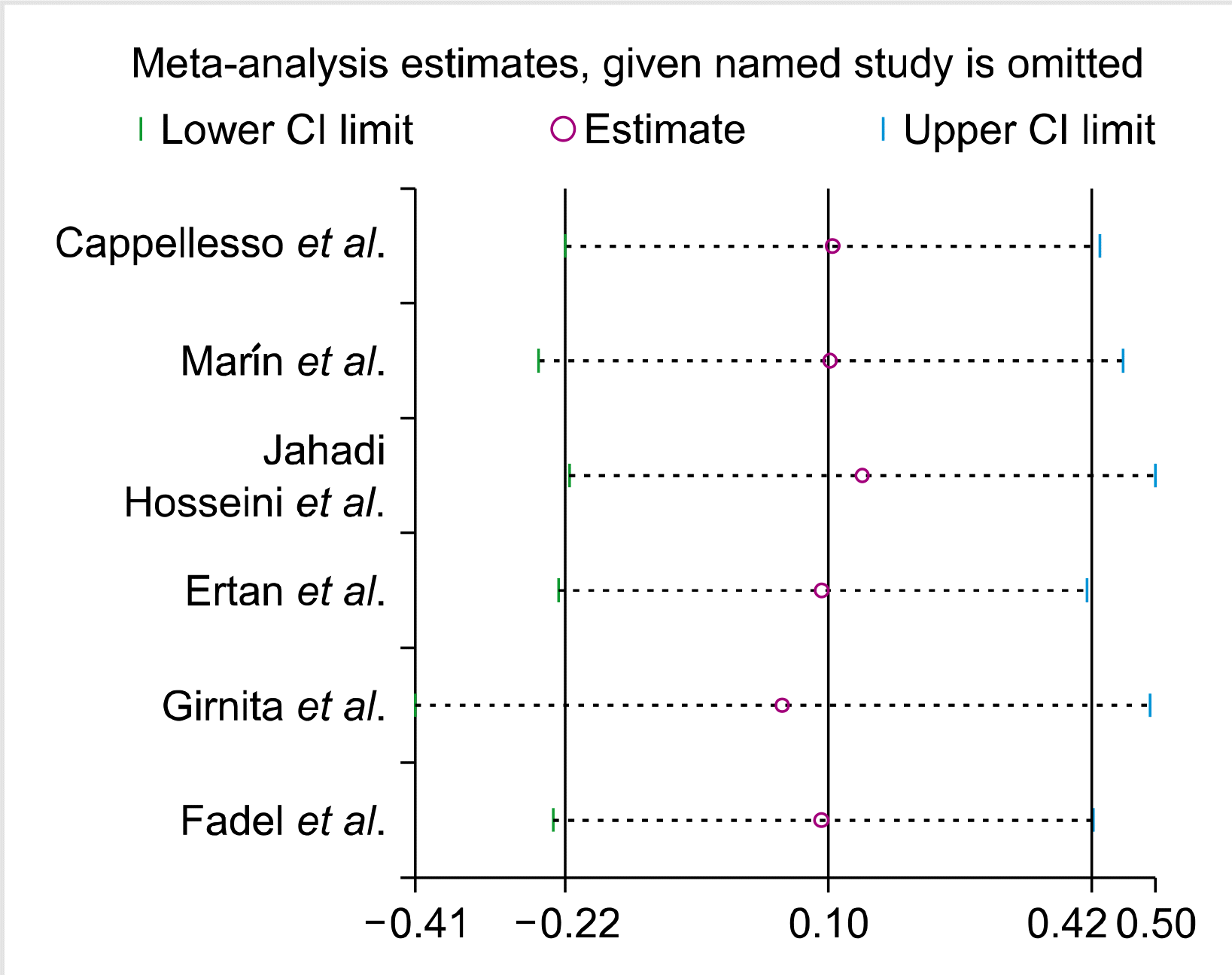
Table 1
Characteristics of studies included in meta-analysis of overall FAS-670A>G.
| Study author | Year | Country | Ethnicity | Sex cases/controls | Total cases/control | Age case/control (mean) | Genotyping method | Quality score |
|---|---|---|---|---|---|---|---|---|
| Cappellesso et al. [24] | 2002 | France | Caucasian | M=NR | 20/77 | NR/NR | RFLP-PCR | 6 |
| F=NR | ||||||||
| Marín et al. [25] | 2006 | Spain | Caucasian | M=NR | 53/227 | 49±12/NR | RFLP-PCR | 7 |
| F=NR | ||||||||
| Jahadi Hosseini et al. [26] | 2009 | Iran | Caucasian | M=NR | 47/225 | 43.67±22.18/40.08±22.18 | ASO-PCR | 7 |
| F=NR | ||||||||
| Ertan et al. [16] | 2010 | Turkey | Caucasian | M=NR | 16/37 | 12.3±0.6/12.3±0.6 | RFLP-PCR | 7 |
| F=NR | ||||||||
| Girnita et al. [27] | 2011 | Multicenter | Mixed | M=NR | 124/405 | NR/NR | PCR | 6 |
| F=NR | ||||||||
| Fadel et al. [28] | 2016 | Egypt | Arab | M=10/19 | 17/30 | 9.37±3.56/10.09±2.95 | RFLP-PCR | 8 |
| F=7/11 |
Table 2
Distribution of genotype and allele among FAS 670A/G patients and controls.
| Study author | Rejection cases | Non-rejection control | P-HWE | MAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| AA | AG | GG | A | G | AA | AG | GG | A | G | ||||
| Cappellesso et al. [24] | 8 | 9 | 3 | 25 | 15 | 25 | 40 | 12 | 90 | 64 | 0/54 | 0/415 | |
| Marín et al. [25] | 15 | 24 | 14 | 54 | 52 | 65 | 106 | 56 | 236 | 218 | 0/33 | 0/48 | |
| Jahadi Hosseini et al. [26] | 12 | 20 | 15 | 44 | 50 | 77 | 73 | 75 | 227 | 223 | ≤0.001 | 0/495 | |
| Ertan et al. [16] | 4 | 10 | 2 | 18 | 14 | 11 | 23 | 3 | 45 | 29 | 0/06 | 0/391 | |
| Girnita et al. [27] | 40 | 46 | 38 | 126 | 122 | 81 | 213 | 111 | 375 | 435 | 0/24 | 0/537 | |
| Fadel et al. [28] | 4 | 3 | 10 | 11 | 23 | 11 | 3 | 16 | 25 | 35 | ≤0.001 | 0/583 | |
Table 3
Main results of pooled OR in meta-analysis of FAS 670A/G gene polymorphisms.
| Genetic model | Sample size | Test of association | Test of heterogeneity | Test of publication bias (Begg’s test) | Test of publication bias (Egger’s test) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||
| Case/control | OR | 95% CI (P) | I2 (%) | P | Z | P | T | P | ||||||
| Overall population | Dominant model | 277/1001 | 0.81 | 0.58–1.12 (0.19) | 33.2 | 0.18 | 0.94 | 0.34 | 1.8 | 0.14 | ||||
| Recessive model | 277/1001 | 1.10 | 0.80–1.53 (0.55) | 0 | 0.99 | 0.19 | 0.85 | 0.14 | 0.89 | |||||
| Allelic model | 277/1001 | 0.96 | 0.79–1.18 (0.7) | 0 | 0.74 | 1.69 | 0.09 | 1.81 | 0.14 | |||||
| GG vs. AA | 277/1001 | 0.92 | 0.62–1.36 (0.66) | 0 | 0.78 | 0.56 | 0.57 | 1.8 | 0.14 | |||||
| AG vs. AA | 277/1001 | 0.75 | 0.52–1.08 (0.12) | 48 | 0.08 | 0.94 | 0.34 | 1.66 | 1.17 | |||||
| Subgroup analysis | ||||||||||||||
| Caucasians | Dominant model | 136/566 | 1.12 | 0.71–1.78 (0.62) | 0 | 0.74 | 0 | 1 | -0.35 | 0.76 | ||||
| Recessive model | 136/566 | 1.02 | 0.63–1.66 (0.93) | 0 | 0.97 | 1.36 | 0.17 | 0.86 | 0.48 | |||||
| Allelic model | 136/566 | 1.06 | 0.80–1.41 (0.67) | 0 | 0.91 | 0 | 1 | -0.36 | 0.75 | |||||
| GG vs. AA | 136/566 | 1.15 | 0.64–2.06 (0.64) | 0 | 0.94 | 0.68 | 0.49 | 0.05 | 0.96 | |||||
| AG vs. AA | 136/566 | 1.15 | 0.70–1.89 (0.58) | 0 | 0.64 | 0 | 1 | -0.38 | 0.74 | |||||
| Children | Dominant model | 157/472 | 0.62 | 0.40–1.06 (0.07) | 37.7 | 0.20 | 1.57 | 0.11 | 5.15 | 0.12 | ||||
| Recessive model | 157/472 | 1.19 | 0.77–1.84 (0.43) | 0 | 0.96 | 1.57 | 0.11 | 2.79 | 0.21 | |||||
| Allelic model | 157/472 | 0.90 | 0.68–1.17 (0.42) | 0 | 0.43 | 1.57 | 0.11 | 4.79 | 0.13 | |||||
| GG vs. AA | 157/472 | 0.79 | 0.47–1.33 (0.36) | 0 | 0.48 | 0.52 | 0.60 | 2.29 | 0.21 | |||||
| AG vs. AA | 157/472 | 0.72 | 0.27–1.95 (0.52) | 40 | 0.18 | 1.57 | 0.11 | 53.19 | 0.01 | |||||
| Adults | Dominant model | 100/452 | 1.22 | 0.72–2.07 (0.47) | 0 | 0.45 | 1.0 | 0.31 | * | * | ||||
| Recessive model | 100/452 | 1.01 | 0.60–1.69 (0.98) | 0 | 0.76 | 1.0 | 0.31 | * | * | |||||
| Allelic model | 100/452 | 1.09 | 0.79–1.51 (0.60) | 0 | 0.76 | 1.0 | 0.31 | * | * | |||||
| GG vs. AA | 100/452 | 1.17 | 0.62–2.22 (0.62) | 0 | 0.79 | 1.0 | 0.31 | * | * | |||||
| AG vs. AA | 100/452 | 1.27 | 0.71–2.28 (0.41) | 0 | 0.33 | 1.0 | 0.31 | * | * | |||||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download