INTRODUCTION
According to the current world health organization (WHO) classification, mature B-cell neoplasms involve a myriad of LPDs, whose presentation and progression vary from indolent to aggressive. Several imaging studies and laboratory tests are usually performed prior to initiation of therapy for staging and prognosis purposes [
1].
For the laboratory testing, the cornerstone remains the lymph node biopsy, since more than two-thirds of lymphoma cases present with peripheral lymphadenopathy [
2]. Equally important is determining the bone marrow involvement because it is the most common site of extra-nodal involvement in lymphoid malignancies and represents a disseminated disease. In some cases, it is the only accessible site or, in rare situations, the only site of involvement [
2]. Since radiological investigations cannot fully assess the degree or pattern of involvement, confirmed infiltration is only possible via pathological examination. However, in the cases where bone marrow morphology is inconclusive, due to focal infiltration or an increased number of reactive cells, immuno-histochemistry is the only tool that can be used for a definite diagnosis [
2].
RECAF is a receptor present on the surface of fetal cells and in the cytoplasm. It binds and internalizes the circulating major fetal serum protein, alpha fetal protein (AFP), which is synthesized in the fetus by the yolk sac and liver, and then declines after birth, being absent in adults [
3,
4]. While the AFP is a well-known tumor marker in hepatic and testicular carcinoma cases, RECAF is a newly emerging broad-spectrum tumor marker [
5]. AFP in prenatal life, like albumin in adults, transports molecules intracellularly via its receptor [
6,
7]. The uptake of AFP and, hence, the expression of its receptor (RECAF) is closely related to the degree of cell differentiation. It is maximally expressed in immature undifferentiated fetal cells and tissues [
5].
In vitro and
in vivo studies have shown that malignant cells regain the ability to uptake AFP via its receptor (RECAF). RECAF expression was identified in several malignant cell lines, such as breast, colon, lung, and rectal carcinoma cells, but was absent from normal adult tissues [
8-
13]. RECAF is considered an oncofetal antigen with cancer diagnosis, screening, and follow-up potential [
5].
To our knowledge, the expression of RECAF in patients with non-Hodgkin lymphoma has not yet been evaluated. This study aimed to explore the expression of RECAF in LPD patients and its possible correlations with clinical and laboratory parameters.
Go to :

MATERIALS AND METHODS
The current study is a retrospective study carried out using 200 archival bone marrow trephine biopsy specimens. They were subdivided into three major groups.
Group (I) or normal control (NC)
This group included 60 archival BM trephine biopsies free of malignant diseases. These patients have done bone marrow biopsies as a part of their work-up for evaluating thrombocytopenia, hypersplenism, anemia of chronic disease, or aplastic anemia. All of them were proved to have no malignant diseases and reached a final benign final diagnosis.
Group (II) or pathological control (PC)
This group included 38 cases (8 CML cases, 4 cases of yolk sac tumors, 8 cases of neuroblastoma, 9 ALL cases, and 9 multiple myeloma cases).
Group (III) or LPD patients
This group included 102 archival bone marrow trephine biopsies of B-LPD patients (44 DLBCL cases, 22 FL cases, 10 MCL cases, 14 CLL cases, and 12 HCL cases).
Informed verbal consent was obtained from all subjects at the time of initial sampling. All procedures performed in our study were performed in accordance with the ethical standards of the institutional and national research committee. The study procedures respected the ethical standards in the Declaration of Helsinki and its later amendments.
Diagnostic workup of B-LPD
Data retrieved from the medical files of patients included:
Clinical data, including full clinical history, physical examination, staging (according to the Ann Arbor staging system), and allocation into risk groups (the International Prognostic Factors Project for NHL);
Laboratory data, including complete blood count (CBC) (MaxM, Coulter, Boston, MA, USA), the differential white blood cell count, chemical profile (liver and kidney function tests, LDH, uric acid) (Synchron 7X, Beckman Coulter, Brea, CA, USA), cerebro-spinal fluid (CSF) analysis (if needed), lymph node histopathological examination and immuno-histochemical staining, histopathological examination of bone marrow aspiration and trephine biopsies, and flow cytometry immunophenotype to establish clonality (Epics II, Beckman Coulter, Brea, CA, USA);
Radiological data, including chest X-ray and/or Computed tomography (CT), abdomen/pelvis ultrasound, PET scan, and magnetic resonance imaging (MRI), when indicated;
Detection of bone marrow infiltration was confirmed in all cases using immunohistochemistry after an initial histopathological examination of bone marrow samples by performing at least CD20/CD3 analysis and evidence of clonality (kappa/lambda).
Detection of RECAF using immunohistochemistry (IHC)
Tissue cuts, 4–6-mm thick, were obtained from blocks of archival formalin-fixed/paraffin-embedded BM trephine biopsies and applied on positively charged slides.
Deparaffinization was performed through passage in xylene (×3, 5 min each). Rehydration of the sections was done using a graded alcohol series (×2, 10 min each in 100%, followed by 95% ethanol), followed by an agitated wash in deionized water for 1 min.
Immunohistochemical staining was performed using the avidin-biotin immuno-peroxidase technique. A peroxidase blocker (0.3% hydrogen peroxide and 0.1% sodium azide) was applied for 10–20 min on the tissue area encircled by a diamond pen to inhibit the action of endogenous peroxidase. Incubation overnight at 2–8°C in a humidified chamber with the primary antibody (mouse monoclonal anti-AFP receptor) (Santa Cruz, CA, USA) was followed by incubation for 30 min each with a secondary biotin-conjugated antibody, and then streptavidin-peroxidase conjugate. DAB (freshly prepared) was applied for 6–12 min and washed twice in phosphate buffer saline (PBS) 7% after each step.
Counterstaining was performed in hematoxylin for a maximum of 30–60 s. Dehydration (in increasing grades of ethanol) was followed by cleaning with xylene, and mounting.
Controls were used for each run (for validity and quality): positive from a placental tissue and negative, in which the primary antibody was replaced by non-immune serum.
Interpretation of results
At least 100–200 cells per smear were counted by two experienced hemato-pathologists to evaluate the number and percentage of RECAF-positive cells.
Statistical methods
Data were analyzed statistically using SPSS version 13. Central tendencies and dispersion [mean (_x) /standard deviation (SD) in parametric data or median/Interquartile (IQ) in non-parametric data] were calculated.
For probability, the chi-square test (X2), Wilcoxon rank sum test, Fisher’s exact test, and Student’s t-test for independent samples were performed. Statistical correlations between data were calculated according to the Ranked Spearman correlation test.
The diagnostic validity test of RECAF, including the cut-off level, sensitivity, specificity, and efficacy were determined using receiver operating characteristic curve (ROC curve) analysis.
The P-values indicating statistical significance and a high statistical significance were lower than 0.05 and 0.01, respectively.
Go to :

RESULTS
See
Fig. 1–
19.
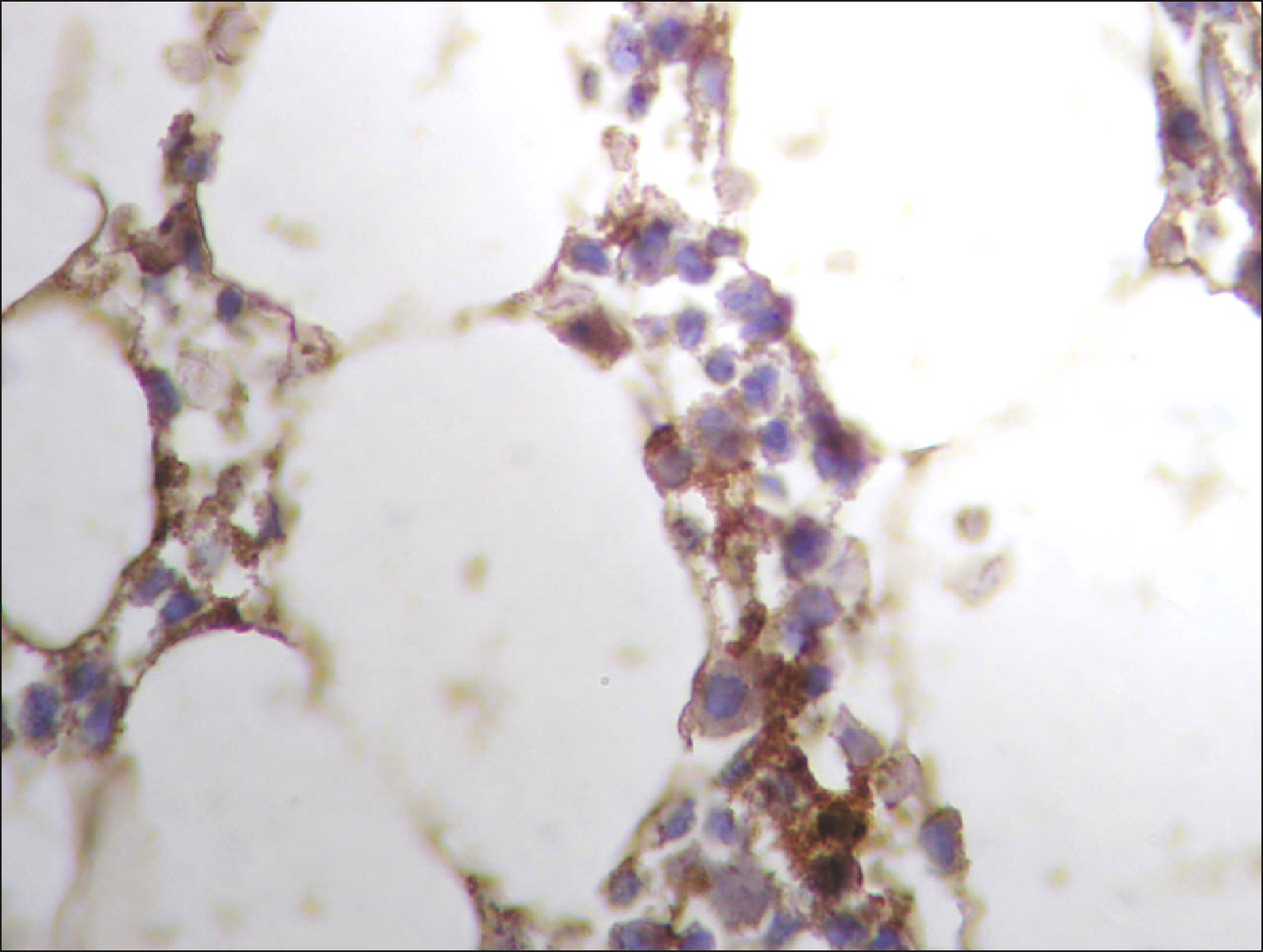 | Fig. 1Diffuse RECAF positive reaction in the majority of cells in DLBCL. 
|
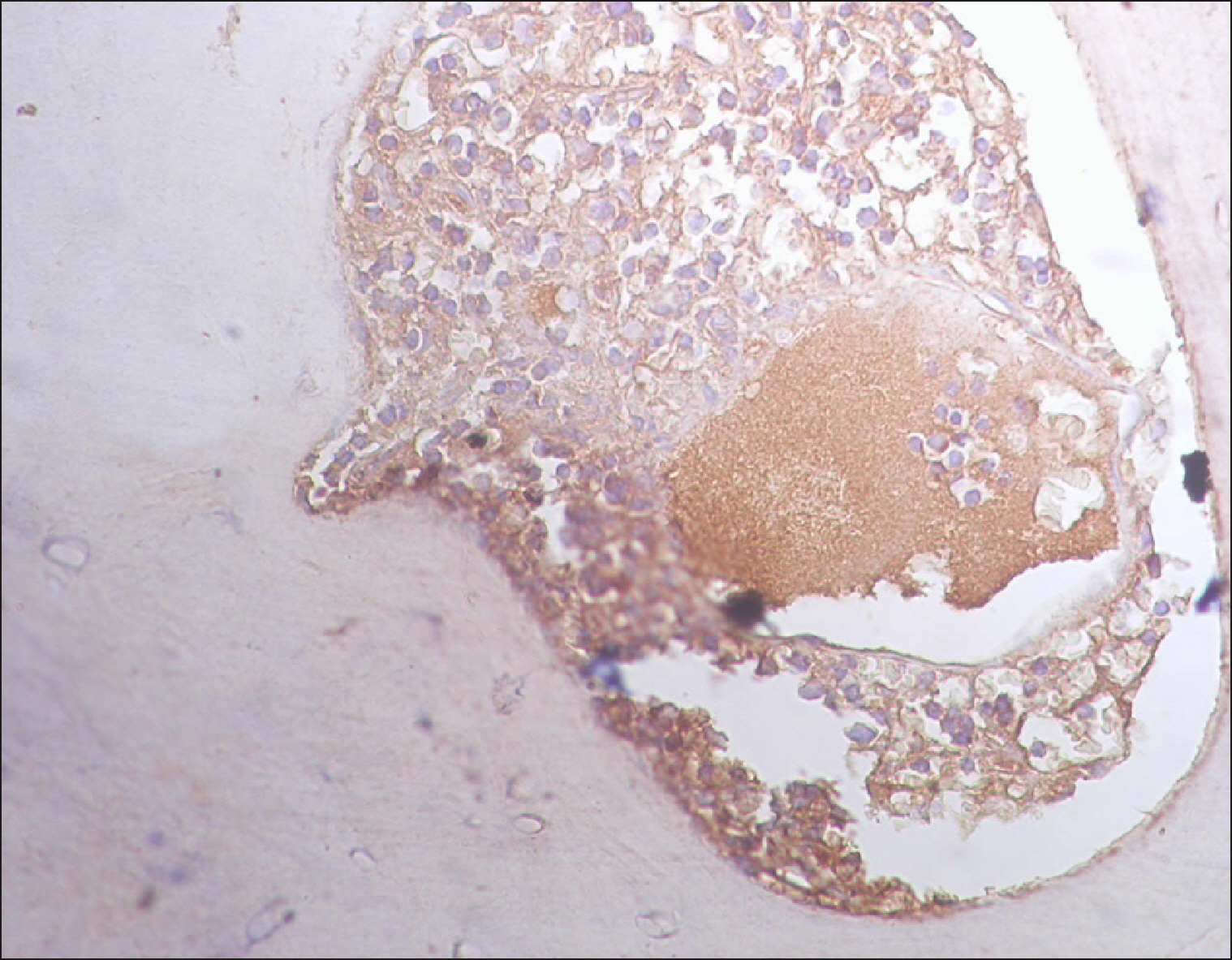 | Fig. 2Diffuse RECAF positivity in DLBCL. 
|
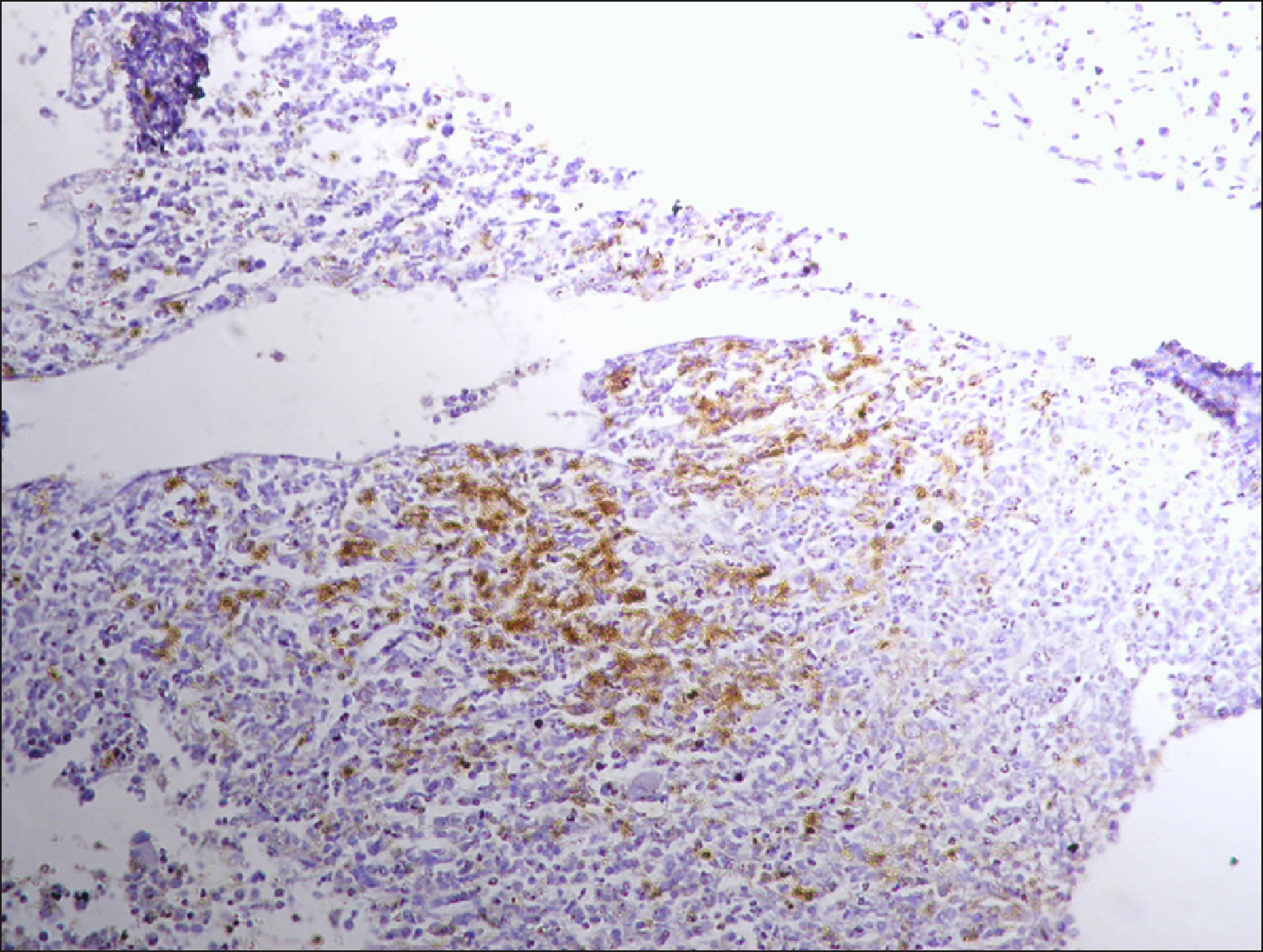 | Fig. 3Collections of RECAF-positive cells in DLBCL. 
|
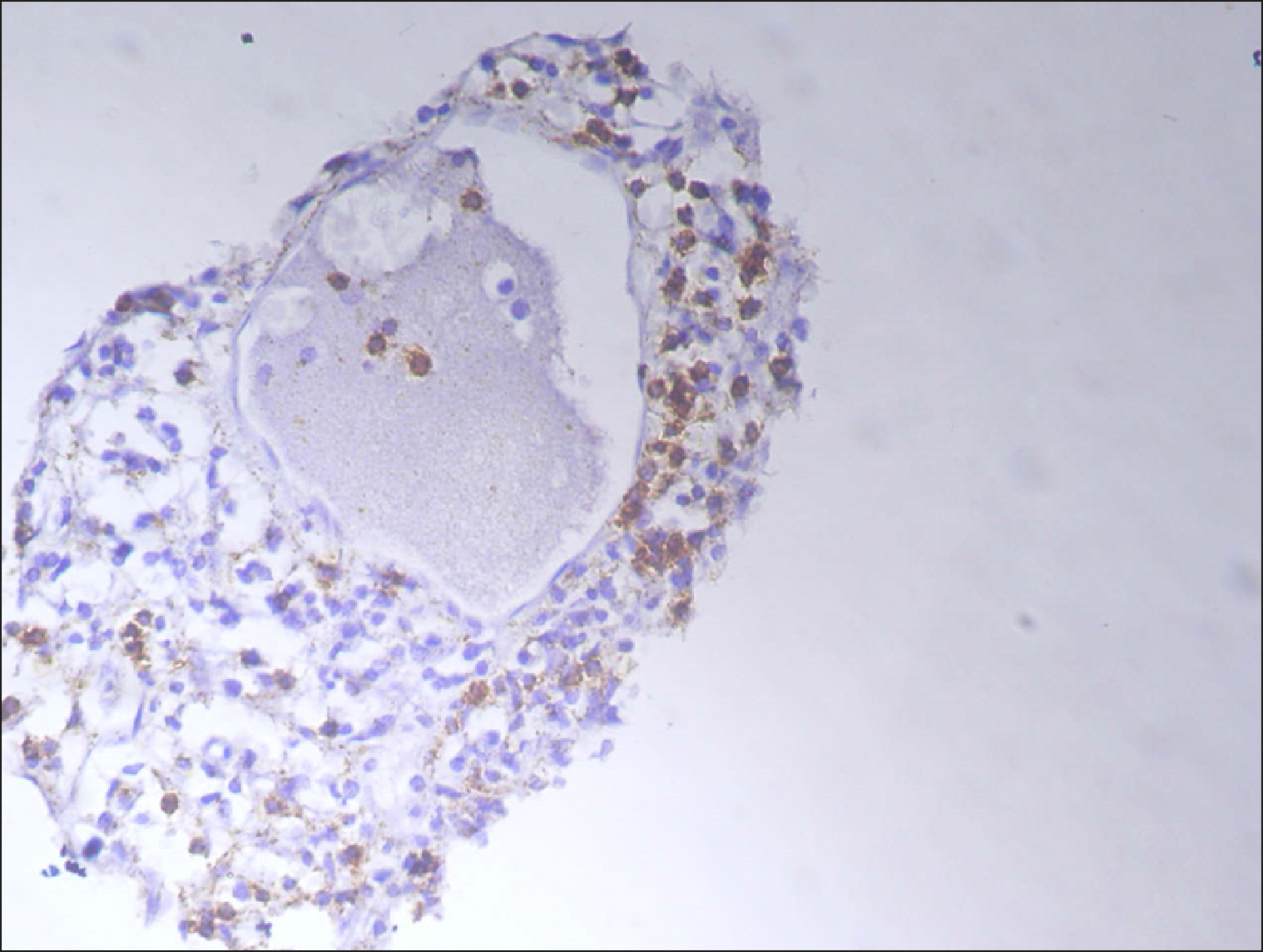 | Fig. 4Sporadic RECAF-positive cells in DLBCL. 
|
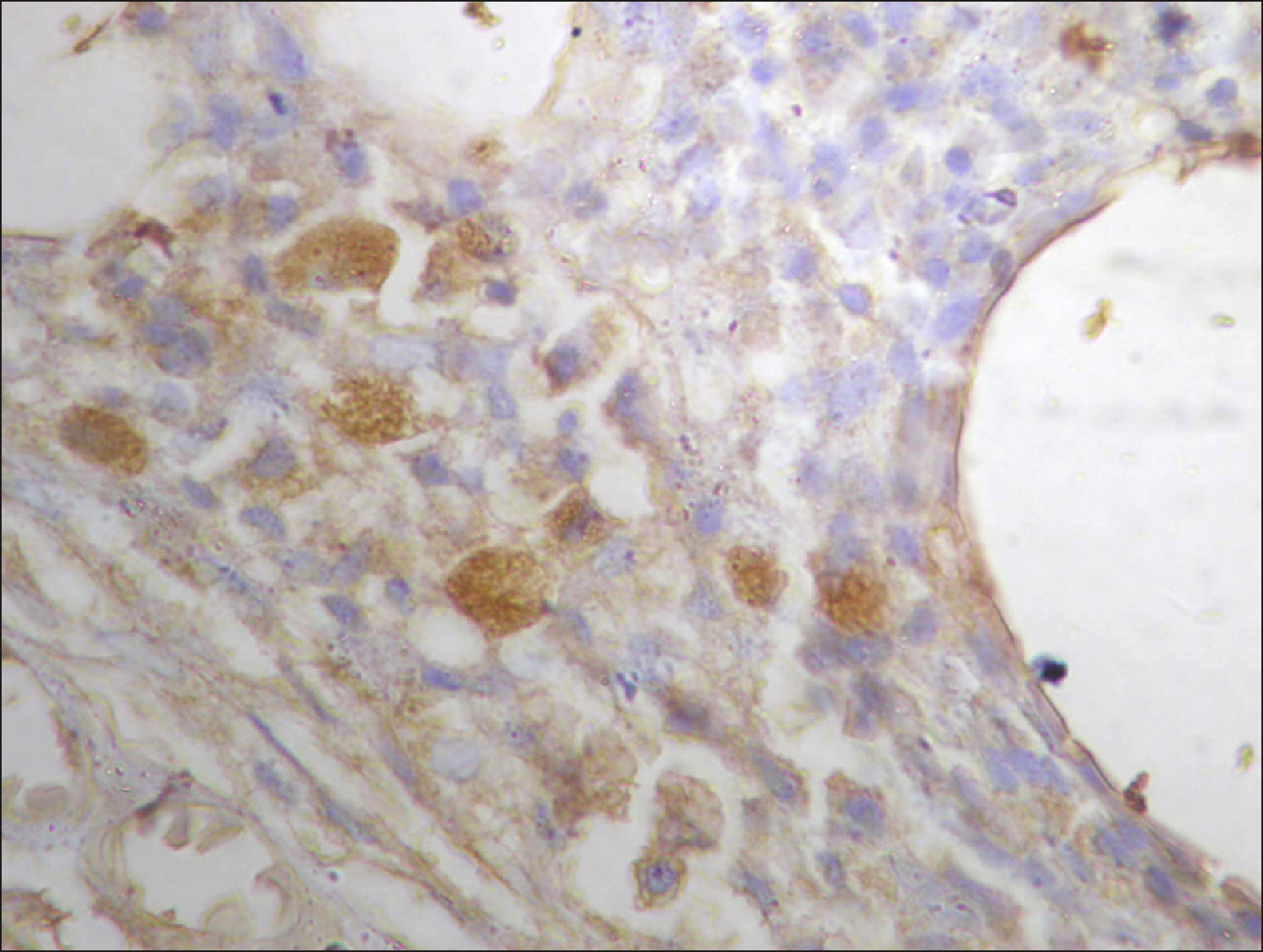 | Fig. 5Many and deeply stained RECAF-positive cells in DLBCL. 
|
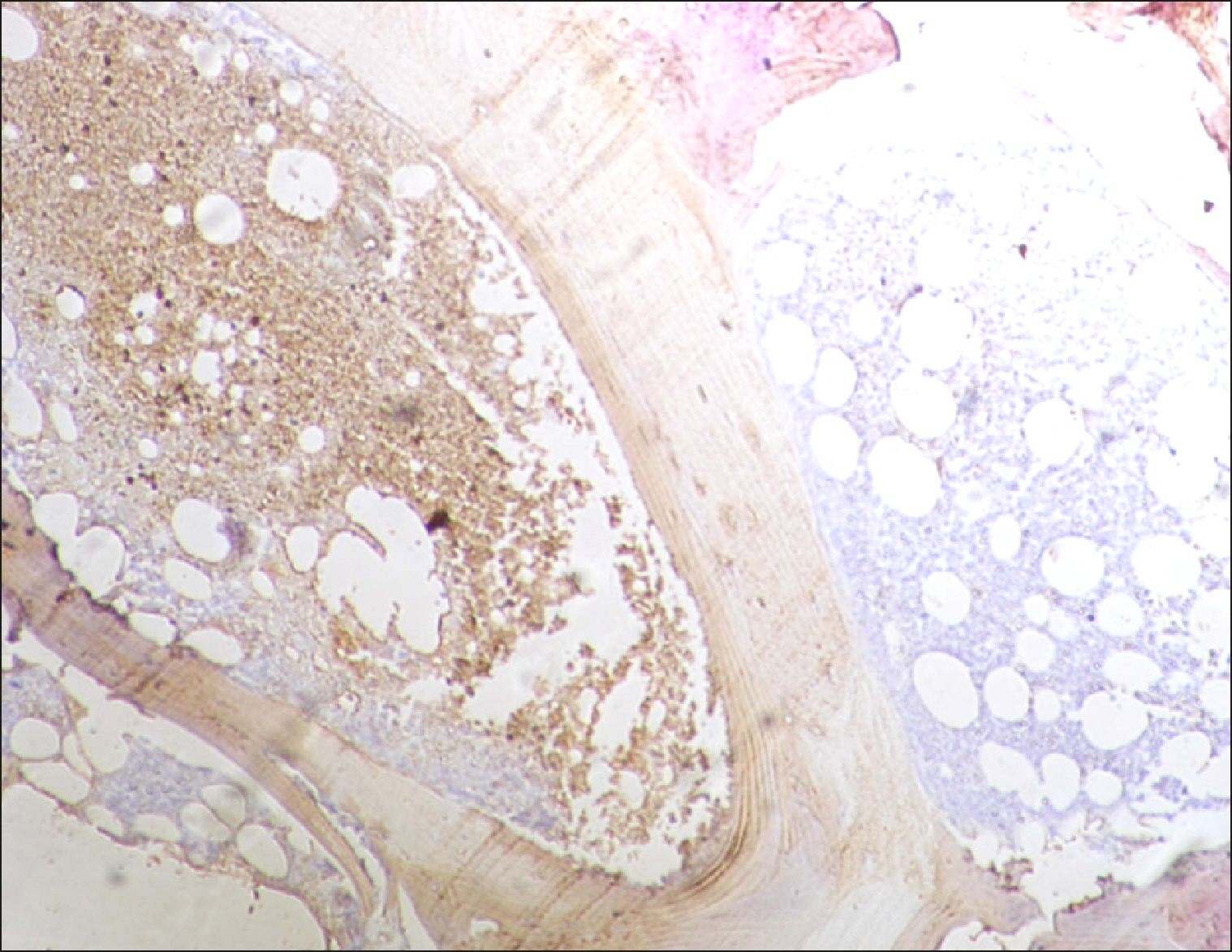 | Fig. 6RECAF-positive follicle (on the left) and a negative follicle (right) in the FL. 
|
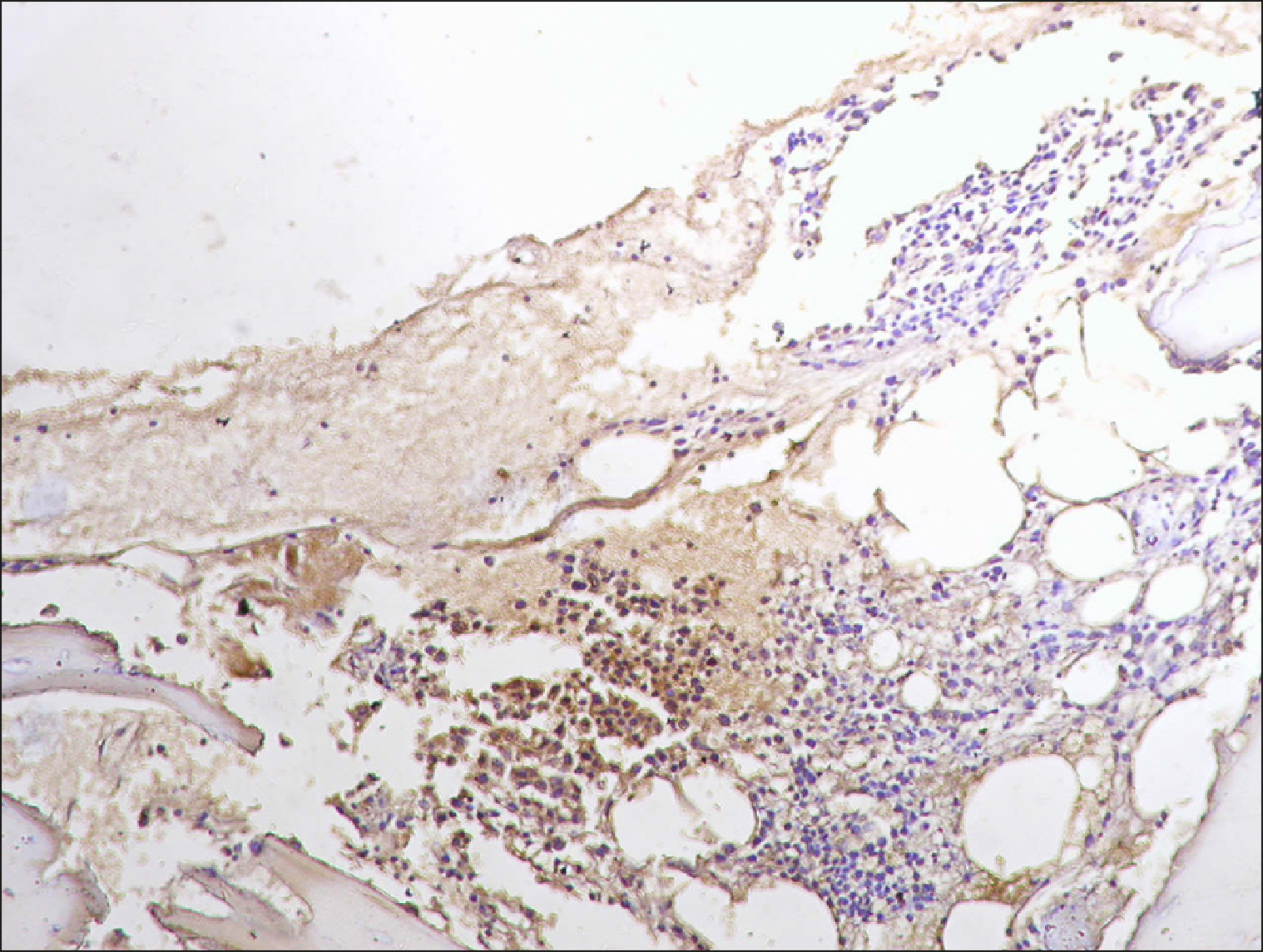 | Fig. 7A collection of RECAF-positive cells surrounded by negative cells in FL. 
|
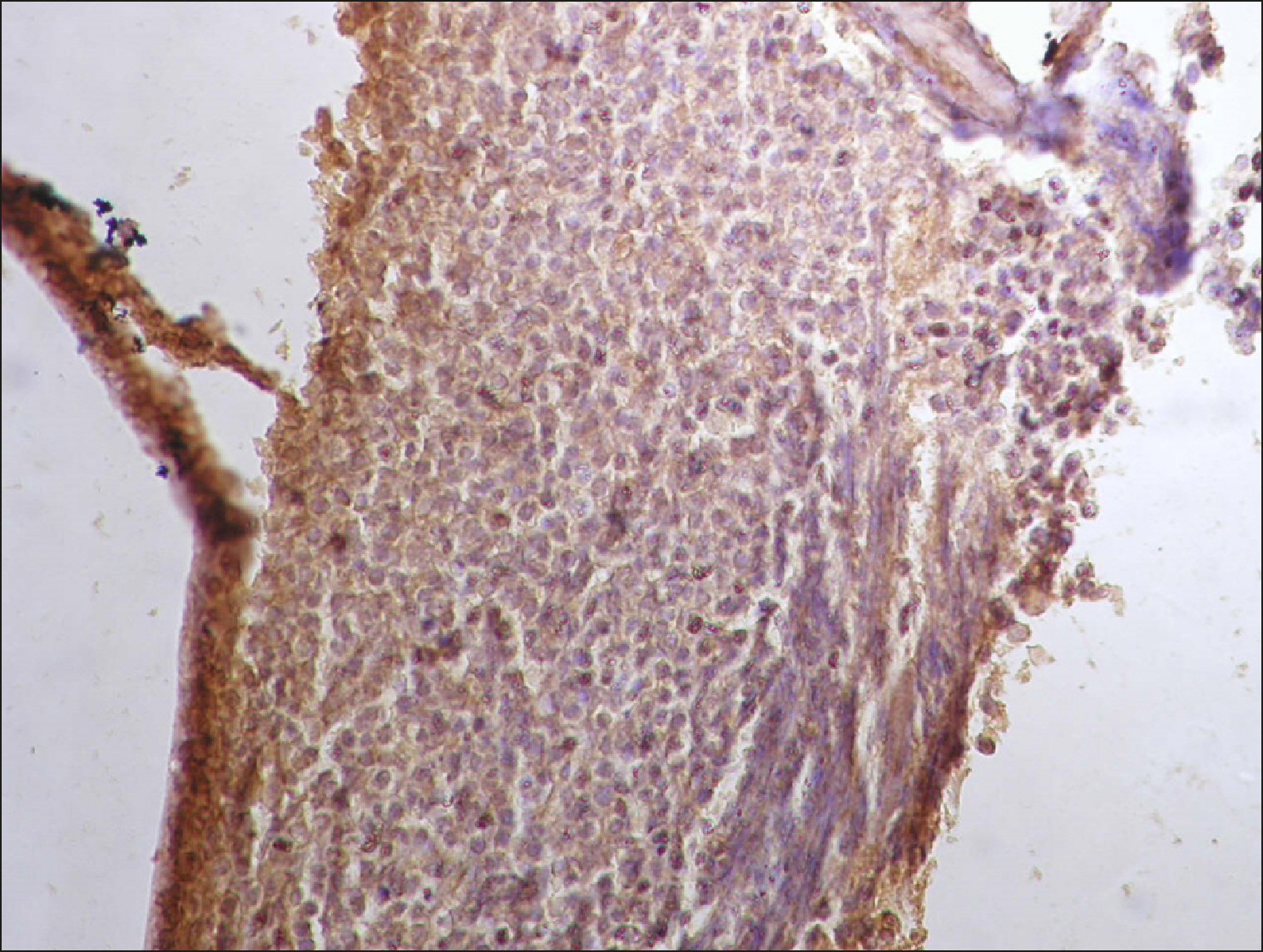 | Fig. 8Diffuse RECAF positivity in FL. 
|
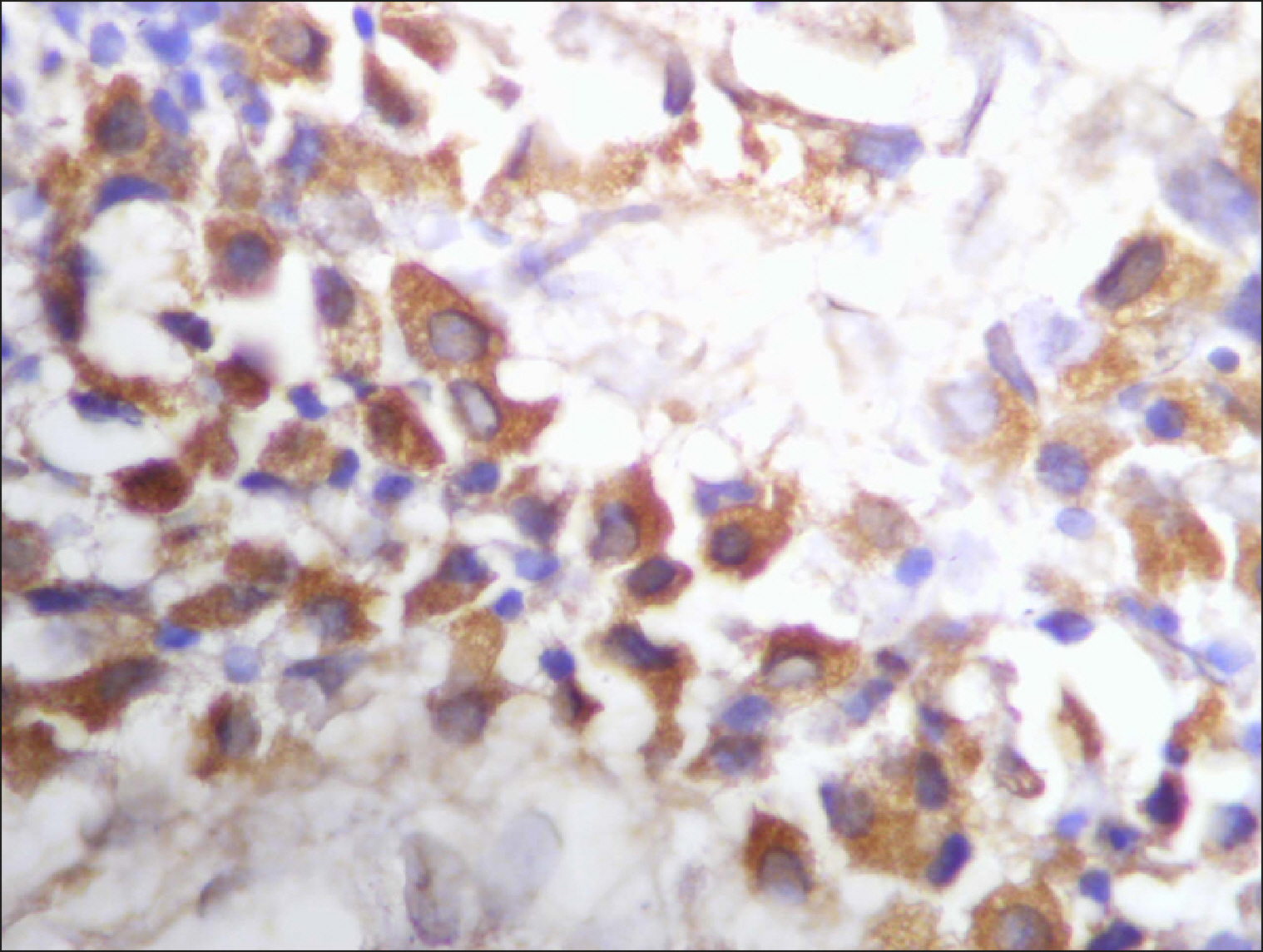 | Fig. 9Many and deeply stained RECAF-positive cells in MCL. 
|
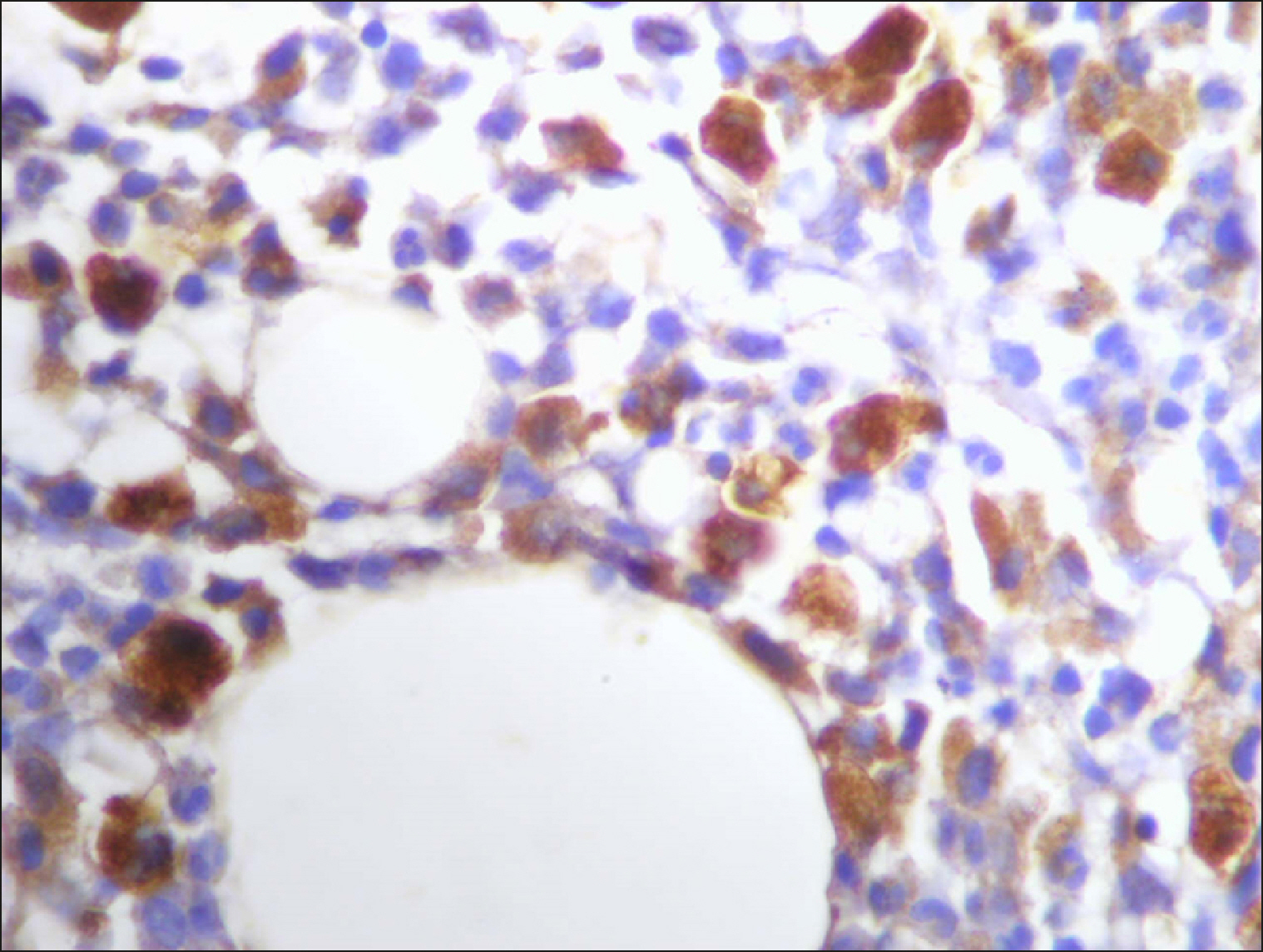 | Fig. 10Many and deeply stained RECAF-positive cells in MCL. 
|
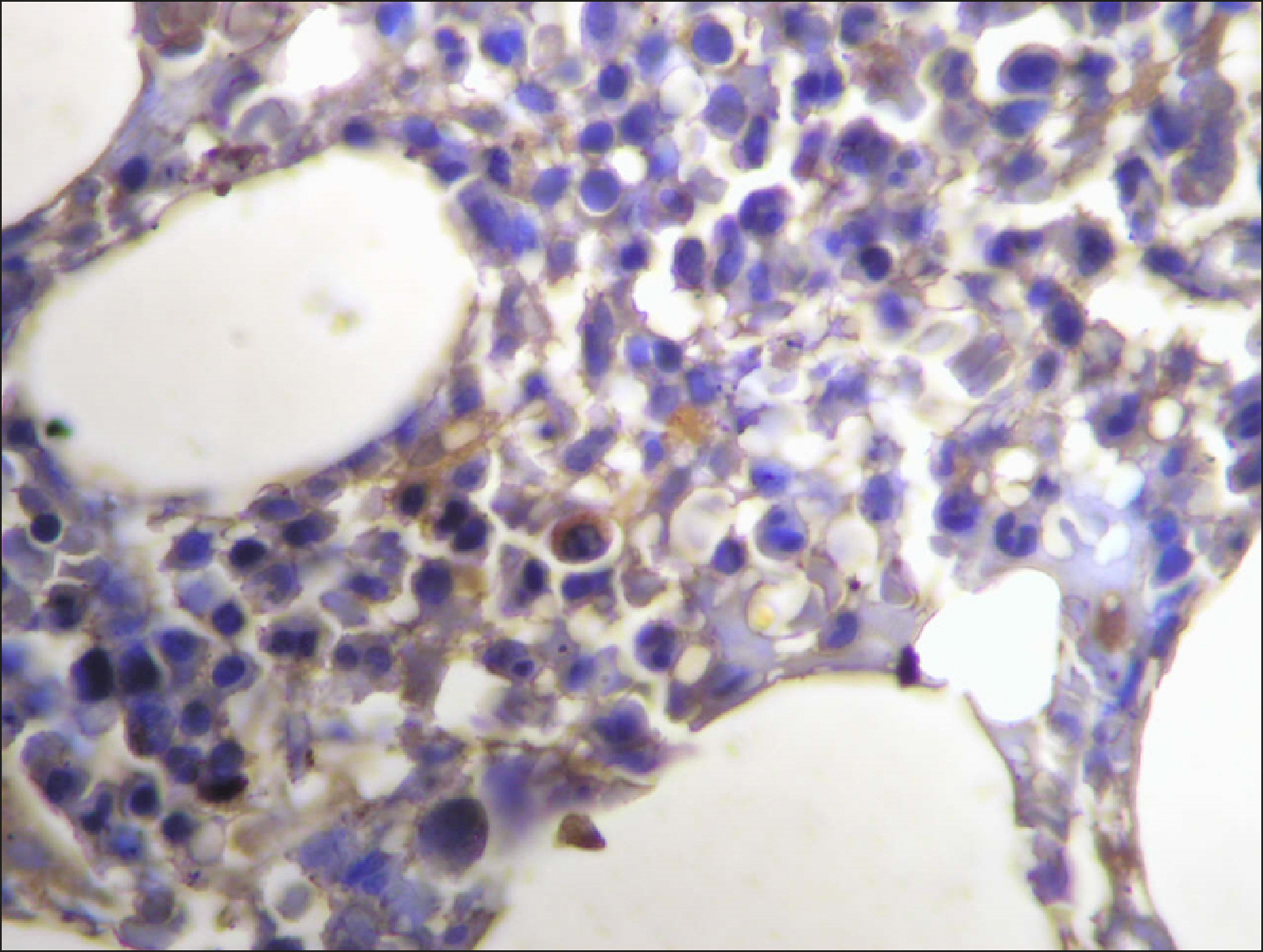 | Fig. 11Scattered RECAF-positive cells in CLL. 
|
 | Fig. 12
|
 | Fig. 13
|
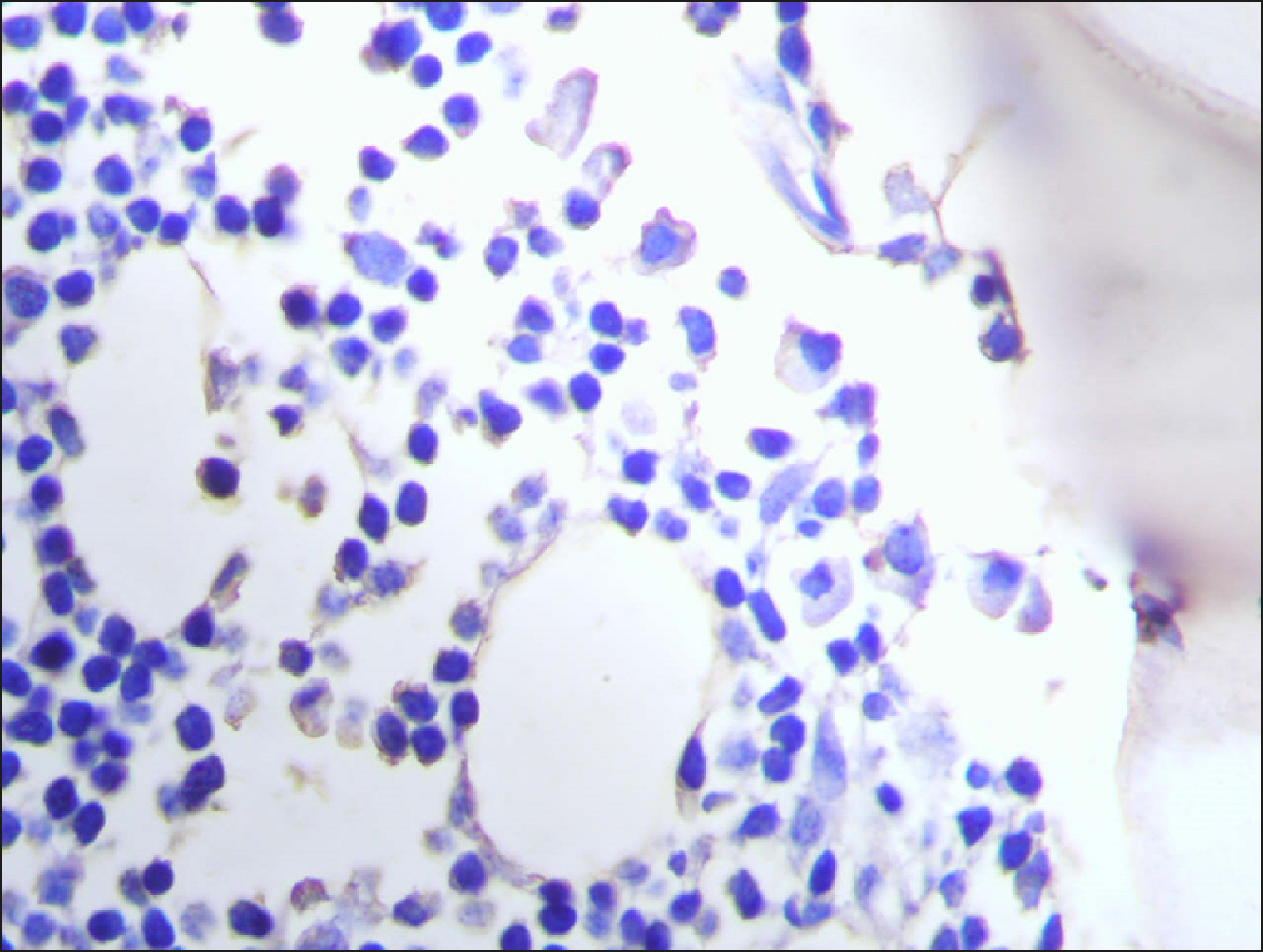 | Fig. 14Sporadic RECAF-positive cells in neuroblastoma. 
|
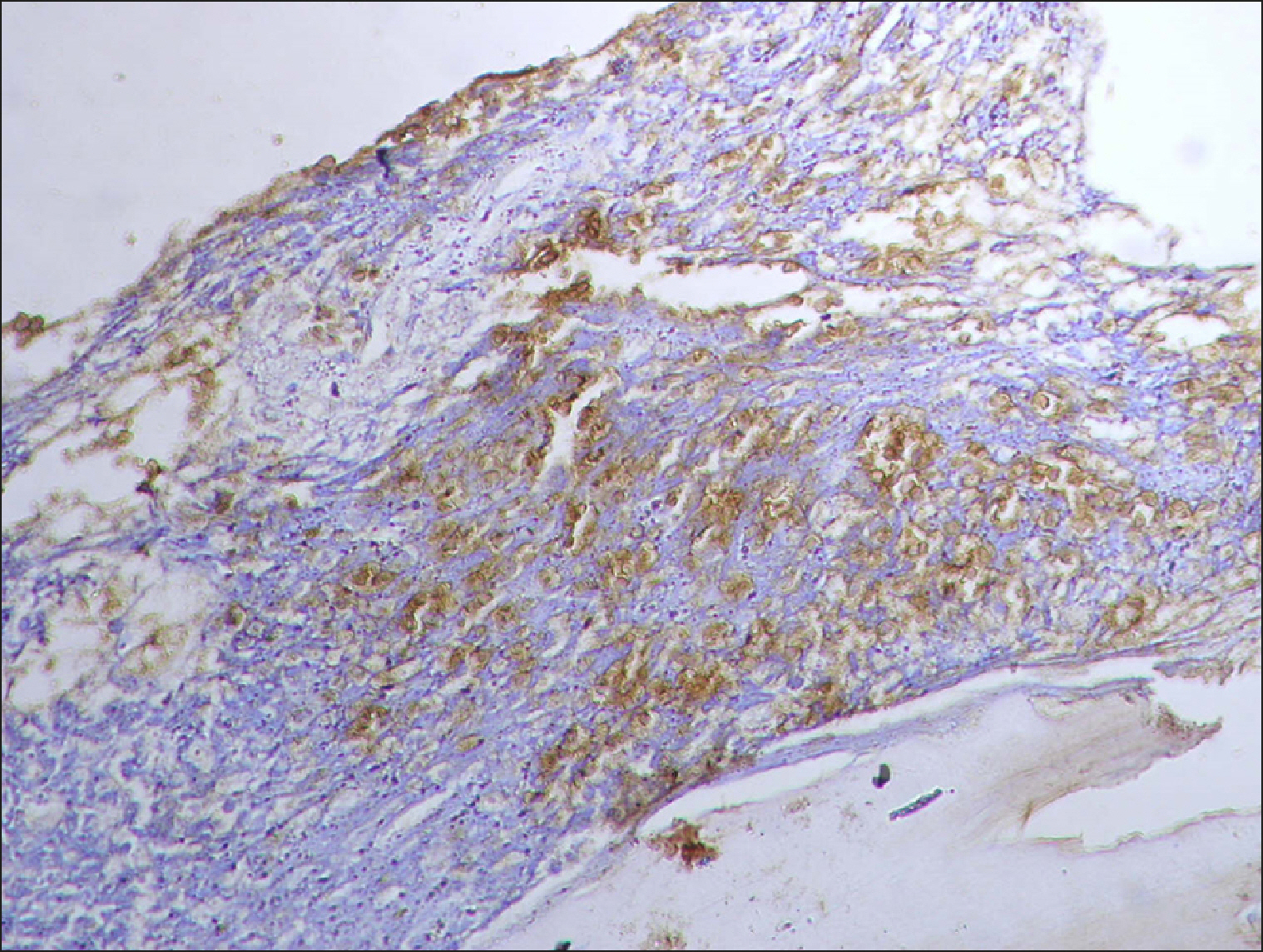 | Fig. 15Collections of RECAF-positive cells in ALL. 
|
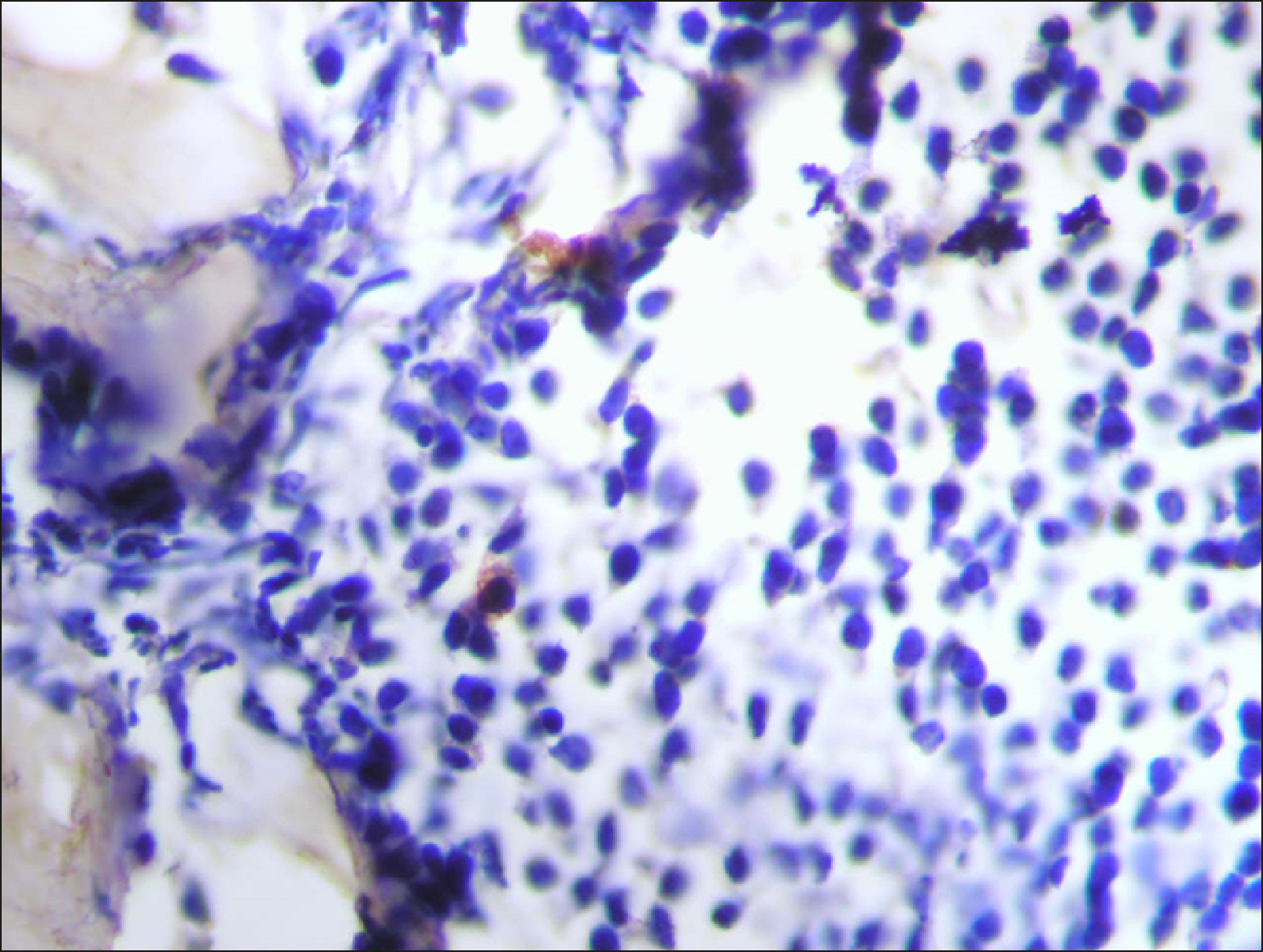 | Fig. 16Few RECAF-positive cells in Multiple Myeloma. 
|
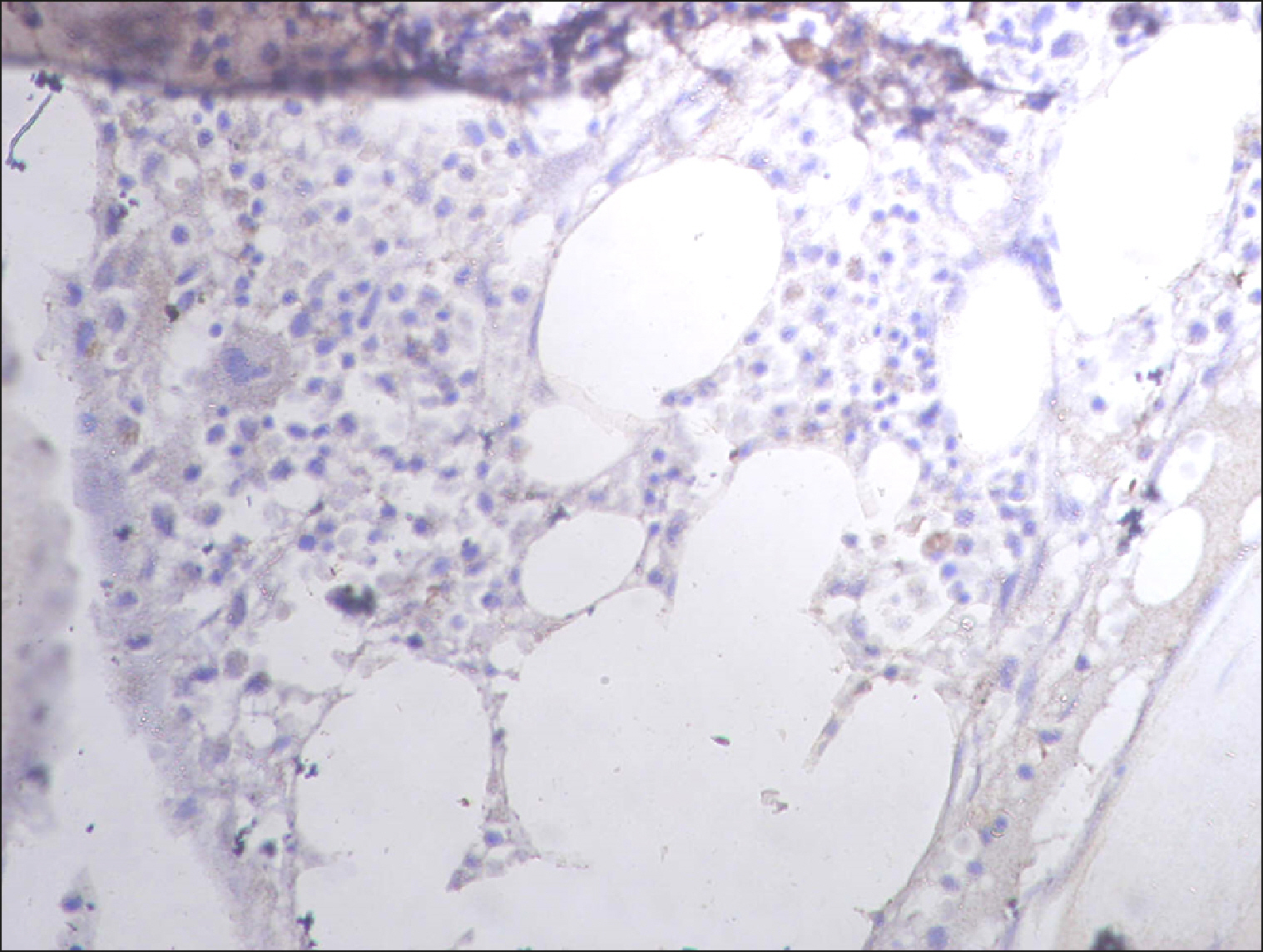 | Fig. 17Sporadic RECAF-positive cells in neuroblastoma. 
|
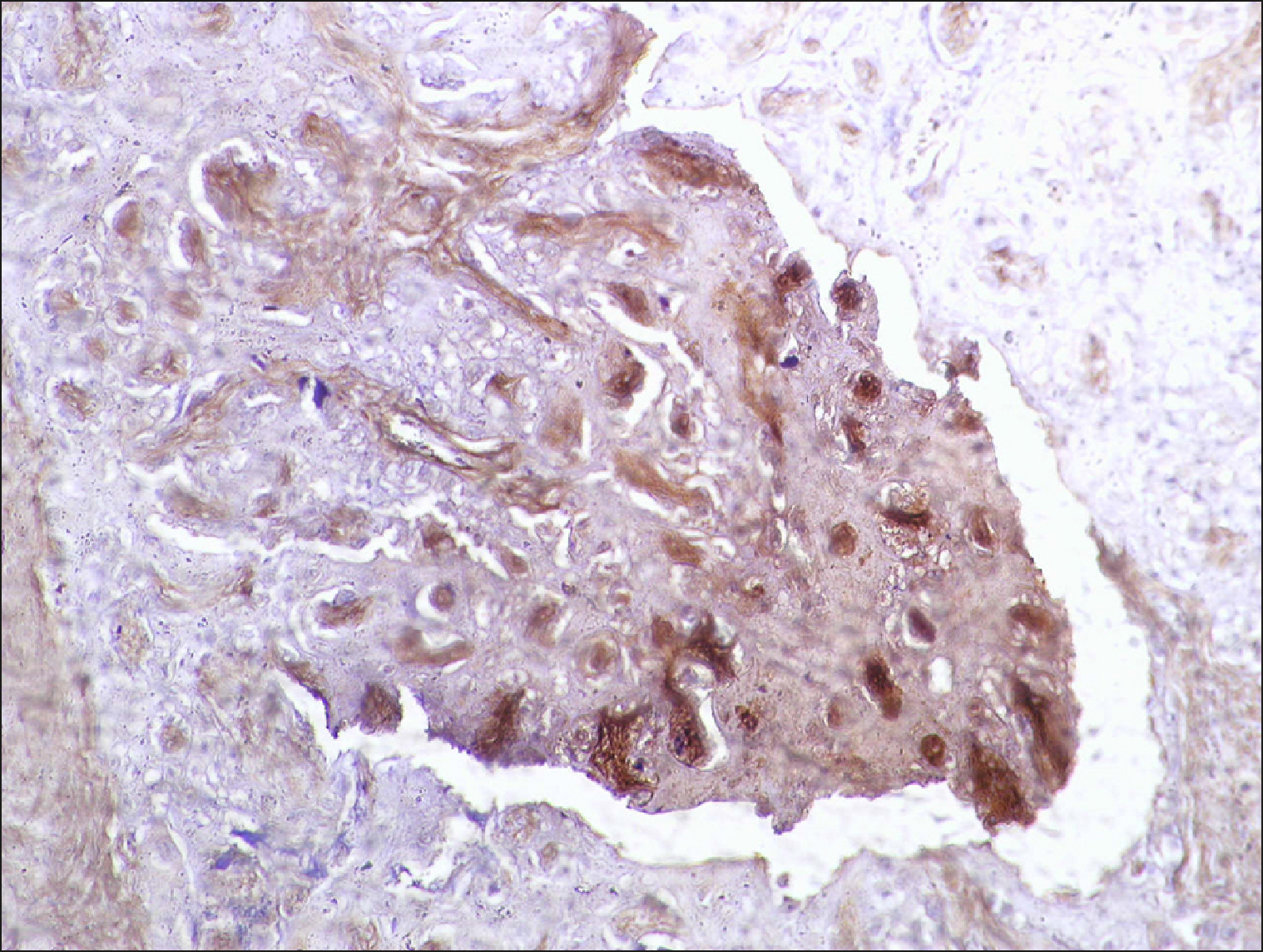 | Fig. 18Placental tissue stained with RECAF (positive control). 
|
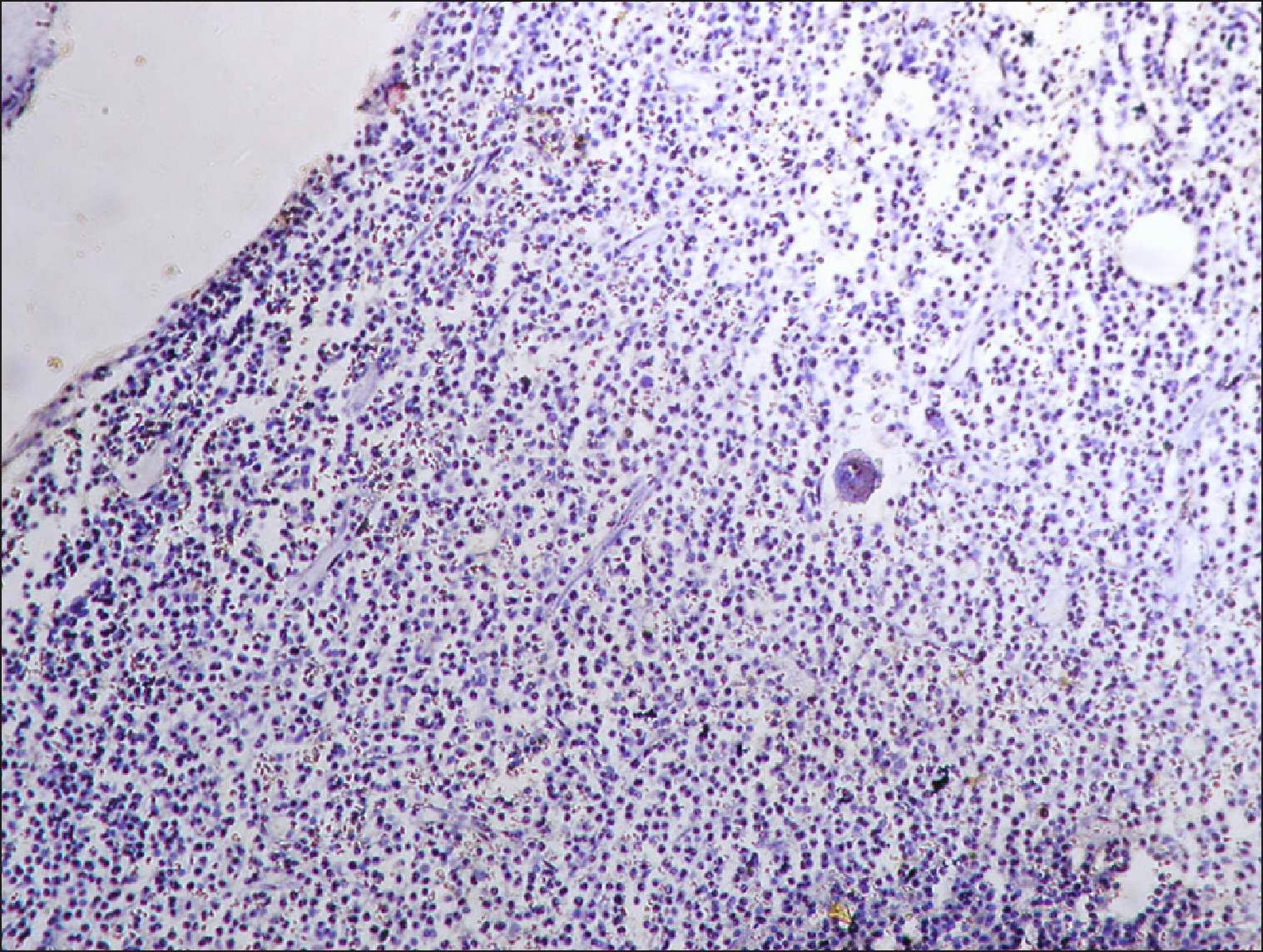 | Fig. 19Normal control negative for RECAF. 
|
Clinical characteristics of the studied groups
The study included 200 archival bone marrow trephine biopsies. Subjects were subdivided into three major groups: normal controls (group I, N=60), pathological controls (group II, N=38), and LPD cases (group III, N=102). Clinical and laboratory findings of the studied groups are presented in
Table 1, while the descriptive data and staging of group III are presented in
Table 2.
Table 1
Descriptive data of studied groups.
|
Parameter |
NC |
PC |
NHL |
|
Age, yr (mean±SD) |
37±7.8 |
24.2±7.3 |
48±11.2 |
|
Gender, male/female |
32/28 |
21/17 |
47/55 |
|
Hb, g/dL (mean±SD) |
11.5±1.4 |
11.5±2.2 |
11.9±1.1 |
|
Platelets, cells×103/mL (mean±SD) |
217±51.3 |
216.8±68.8 |
271.1±86.8 |
|
LDH, mg/dL (mean±SD) |
292±37.7 |
336±70.6 |
540.4±176.7 |
|
Lymphocytes, cells× 103/mL (median and IQ range) |
1.8 (2.2) |
1.9 (23.3) |
2.4 (16.7) |

Table 2
Descriptive data of NHL/CLL cases subgroups included in the study.
|
Parameter |
DLBCL (N=44) |
FL (N=22) |
MCL (N=10) |
CLL (N=14) |
HCL (N=12) |
|
Age, yr (mean±SD) |
43.5±10.8 |
50.6±11.5 |
48±4.3 |
55±8.8 |
52±14.2 |
|
Gender (male/female) |
26/18 |
8/14 |
8/2 |
6/8 |
6/6 |
|
Hb, g/dL (mean±SD) |
12.1±1.2 |
11.8±0.9 |
11.8±0.8 |
11.6±0.3 |
10.2±1.4 |
|
Platelets, cells×103/mL(mean±SD) |
313±76.6 |
245.8±73.8 |
225.6±51.7 |
159.2±9.9 |
106.5±20.3 |
|
LDH, mg/dL (mean±SD) |
555.3±159.6 |
613.8±148.1 |
730±105.1 |
434.7±146.9 |
316.3±44.4 |
|
Lymphocyte count, cells×103/mL (median and IQ range) |
2.2 (2.5) |
2 (2.4) |
2.6 (2.4) |
19 (22) |
2.2(2.5) |
|
B-symptoms, N (%) |
18 (40.9) |
8 (36.4) |
2 (20) |
4 (28.6) |
8 (66.7) |
|
LNS, N (%) |
44 (100) |
22 (100) |
10 (100) |
10 (71.4) |
2(16.7) |
|
HSM, N (%) |
20 (45.5) |
10 (45.5) |
2 (20) |
8 (57.1) |
10 (83.3) |
|
Stage, N (%) |
|
|
|
|
|
|
Stage1 |
8 (18.2) |
2 (9.1) |
0 (0) |
0 (0) |
0 (0) |
|
Stage 2 |
14 (31.8) |
2 (9.1) |
0 (0) |
0 (0) |
0 (0) |
|
Stage 3 |
6 (13.6) |
4 (18.2) |
4 (40) |
0 (0) |
0 (0) |
|
Stage 4 |
16 (36.4) |
14 (63.6) |
6 (60) |
14 (100) |
12 (100) |
|
BM infiltration by histopathology, N (%) |
12 (27.3) |
12 (54.5) |
2 (20) |
14 (100) |
12 (100) |

Expression of RECAF using Immunohistochemistry (IHC), see Table 1 & 3
Five of the 60 normal controls (8%) showed cells that were positive for RECAF expression using IHC. The percentage of RECAF-positive cells in the NC group ranged from 0% to 12%, with a median of 0% and an IQ range of 6%.
The percentage of positive cells in the PC group ranged from 0% to 40% with a median of 6% and an IQ range of 20% (32–40% in ALL and 12–15% in neuroblastoma, but was negative in CML, multiple myeloma, and yolk sac tumors).
The percentage of RECAF-positive cells in DLBCL, FL, MCL, and CLL patients ranged from 0% to 80%, 0% to 90%, 0% to 65%, and 0% to 40%, with a median of 3%, 22%, 7%, and 10%, and an IQ range of 40%, 45%, 32.5%, and 20%, respectively. Meanwhile, all HCL cases were negative for RECAF.
The percentage of RECAF-positive cells was significantly higher in PC and LPD groups compared to the normal control group (P=0.02 and 0.007 respectively). There was no significant difference between NHL patients and the PC group regarding the number of positive cells (P=0.1).
Comparison between LPD subgroups regarding RECAF expression
The percentage of RECAF-positive cells was significantly higher in FL, MCL, DLBCL, and CLL, than in HCL (
P=0.001), with the highest percentage observed in FL and DLBCL (
P=0.001) (
Table 3).
Table 3
Comparison between different B-NHL subgroups as regards percent of RECAF positive cases.
|
Parameter |
DLBCL |
FL |
MCL |
CLL |
HCL |
|
RECAF+ve cases, N (%) |
16/44 (36.1) |
20/22 (90.9) |
8/10 (80) |
6/14 (42.8) |
0 (0) |
|
Fisher exact test |
|
|
P<0.001 |
|
|

Assessment of BM infiltration via routine histopathology
None of the patients in Group I (normal control) showed BM infiltration by malignant diseases. In the pathological control group (group II), all samples were negative for BM infiltration via routine histopathological examination, except for CML, MM, and ALL cases.
Fifty-two of the 102 LPD patients (51%) had BM infiltration, which was detected via routine histopathological examination. These were 12/44 (27.3%) DLBCL, 12/22 (54.5%) FL, 2/10 (20%) MCL, all 14 CLL, and 12 HCL patients (
Table 2).
Cut off percentages of RECAF-positive cells for which BM infiltration was identified
ROC curve analysis of the percentage of RECAF-positive cells in the smears revealed various cut-off values above which the cases were designated as positive for RECAF expression. Accordingly, BM infiltration by RECAF was identified in FL, MCL, DLBCL, and CLL patients at cut-off values of 6%, 5%, 10%, and 15%, respectively (
Table 4).
Table 4
Comparison between RECAF negative and RECAF positive NHL cases as regards laboratory findings.
|
RECAF (cutoff) |
|
|
Negative |
|
Positive |
|
T-test |
|
|
|
|
Mean±SD |
Mean±SD |
t |
P
|
|
Lymphocyt |
6.000±8.532 |
4.100±5.459 |
0.972 |
0.336 |
|
HB |
12.050±1.255 |
11.803±1.068 |
0.752 |
0.456 |
|
Platelet |
273.650±91.468 |
269.516±85.281 |
0.164 |
0.870 |
|
LDH |
597.000±143.546 |
503.903±188.375 |
1.883 |
0.066 |
|
Age |
47.900±10.959 |
48.194±11.626 |
-0.090 |
0.929 |

Role of RECAF expression in the detection of BM infiltration versus routine IHC
Among the PC group, although IHC was more sensitive than routine histopathology for the detection of BM infiltration, the difference was not statistically significant (P=0.9).
Regarding CLL and HCL, the detection of BM infiltration was significantly higher using histopathology 26/26 (100%) compared to RECAF 6/26 (23%) (P=0.001). In FL, MCL, and DLBCL, the detection of RECAF using IHC was more sensitive than routine histopathology for the detection of BM infiltration (P=0.01).
Go to :

DISCUSSION
Bone marrow examination in lymphoproliferative diseases is essential for diagnosis, staging, and prognostic evaluation. Immunohistochemical studies help to reach a definite diagnosis via a variety of B cell or T cell lineage markers. RECAF is a receptor for alpha fetoprotein, that serves as a broad-spectrum tumor marker.
The current study is a retrospective study carried out using 200 archival bone marrow trephine biopsy specimens, including specimens from normal control (NC), pathological control (PC), and LPD patients. In our study, the number of RECAF-positive cells was significantly higher in LPD patients than in NC patients (P=0.007). To our knowledge, this is the first study to investigate the expression of RECAF in NHL/CLL patients using IHC. We document for the first time that RECAF was significantly higher in LPD patients than in normal controls. This will open the door for further evaluation of a possible role of RECAF in the diagnosis, follow-up, detection of minimal residual disease, and detection of relapse in lymphoma patients. Further studies are ongoing at our university.
The uptake of AFP was first documented in fetal cells; it was observed in all three germ layers, ecto-, meso-, and endodermal tissues, whether obtained from human, bird, or mammal embryos [
14-
20]. Later studies showed the capability of muscle tumor cells to internalize exogenous AFP in rhabdomyosarcoma; later in 1984, another study showed the incorporation of AFP by MCF-7 human breast cancer cells [
11,
14,
21]. Then, the same research group proved the existence of a specific membrane receptor for AFP on the surface of MCF-7 human breast cancer cells [
22].
AFP serves as a growth factor in certain cancers, such as hepatocellular carcinomas, to enhance their growth and progression. Immunohistological detection of its receptor (RECAF) has been performed on a variety of tumors, including murine mammary tumors and human adenocarcinoma, human breast, colon, lung, rectal carcinoma, human breast cancer adenocarcinoma, hepatoma, and breast MCF-7 adeno- and ductal carcinoma, breast metastasis [
14,
22-
26]. AFP is thought to regulate neoplastic growth via the presence of its receptor. Following the binding of AFP to its cell surface receptor (RECAF), it undergoes internalization via a receptor-mediated endocytosis process.
As the uptake of AFP and, hence, the expression of RECAF is related to the degree of cell maturation, immature or malignant cells re-express RECAF, while mature adult tissues lack RECAF expression [
5]. In parallel, poorly differentiated normal cells, such as stem cells and reproductive cells, can express RECAF, which may explain the few positive cells observed in the NC group in our study. Also in our study, ALL cases showed the highest level of RECAF positive cells (32–40%) in the PC group. This may be related to the degree of cell maturation, as blast cells are extremely immature. Moreover, negative RECAF cells were observed in patients suffering from diseases that arise from either partially differentiated cells, as in CML, end-stage cells, such as myeloma (plasma) cells, or primitive, but not embryonic cells, as in yolk sac tumors.
Furthermore, in our study, the expression of RECAF was variable among the different subtypes of NHL, with the highest percentage seen in FL and DLBCL (P=0.001). From the results mentioned above; it is evident that the well-known heterogeneity of B-NHL disorders extends to their cellular expression of RECAF. Our results document for the first time the clear distinction between leukemic (DLBCL: FL and MCL) and non-leukemic lymphomas (CLL/SLL and MCL) via RECAF detection. Interestingly, none of HCL cases expressed RECAF. The negativity among all cases of HCL is in itself a positive finding that warrants further confirmatory studies.
No previous studies have investigated the unique pattern of RECAF expression in the different subtypes of lymphoma. However, we believe that RECAF may help in differentiating between different subtypes of lymphoma and in the detection of MRD and relapse. Moreover, RECAF may be a target of treatment in different types of LPD. Further studies are needed to confirm this.
Because detection of BM infiltration is essential in staging LPD patients, we investigated the possible role of RECAF expression in detecting BM infiltration, in comparison to routine histopathology. We found that in FL, MCL, and DLBCL patients, detection of RECAF via IHC was more sensitive than routine histopathology for BM infiltration detection (P=0.01). In contrast, this was not observed in CLL and HCL patients. The added value provided by RECAF detection is the advantage of discriminating malignant from non-malignant lymphocyte populations and not merely assigning the lineage of the lymphoid population. Furthermore, RECAF as a single marker may be easier to perform instead of the full routine IHC panel in the cases already diagnosed via lymph node biopsy when clinicians just need to confirm the stage. Further comparative studies are needed to support our results.
As RECAF serves as a universal tumor marker, ongoing studies are investigating its use for targeting tumor cells [
27]. Since AFP from one species binds to or is uptaken by cells from another species, the cross-reaction of anti-human AFP receptor mabs is a fortunate circumstance that allows the development of simple tumor-targeting experimental models in animals. The interaction between anti-AFP receptor antibodies and malignant cells is not limited to a passive binding but also modulates the rate of cell replication [
5].
Furthermore, RECAF may be used for screening tumors in the future. Another study showed that a RECAF-based serum immunoassay can discriminate breast cancers from normal subjects or benign breast diseases with high sensitivity and specificity. The most valuable finding in this study is that the assay can detect the early stages of breast cancer with high sensitivity and specificity [
5].
RECAF is significantly expressed in the bone marrow of patients with NHL/CLL. Its unique expression pattern may be a promising tool for the diagnosis of different subtypes of NHL. Furthermore, it may help in the detection of BM infiltration in lymphoma cells. This may be the basis for new target therapy for NHL based on RECAF expression. Further studies are needed to confirm this.
Go to :





 PDF
PDF Citation
Citation Print
Print





















 XML Download
XML Download