Abstract
Human parvovirus B19 (HPV-B19) usually infects children. We report a case of an adult with HPV-B19 infection mimicking systemic lupus erythematosus (SLE). A previously healthy 46-year-old woman presented with an acute illness of cough, fever, chilling, polyarthritis, and skin rash. Laboratory findings showed pancytopenia, increased creatinine level, proteinuria and hypocomplementemia. Anti-double stranded DNA antibody (anti-dsDNA Ab) and antinuclear antibody were positive. Highly suspected of SLE based on clinical and laboratory findings, the patient was initially treated with corticosteroids. Meanwhile, the result of HPV-B19 polymerase chain reaction, which was done initially with other viral tests to exclude infection, turned out to be positive. Steroid was tapered, and pancytopenia, proteinuria, hypocomplementemia gradually improved. On the seventh day, anti-dsDNA Ab was found to be negatively converted. HPV-B19 infections are mostly self-limited and occur rarely in adults, but if a patient presents lupus-like syndrome with transient autoantibody positivity, lupus mimickers including HPV-B19 should be considered.
Go to : 
Systemic lupus erythematosus (SLE) is a chronic multi-systemic autoimmune disease, characterized by increased autoreactive cells and autoantibodies. It involves skin, joints, vessels, nerves, and multiple internal organs. However, these manifestations are also found in other diseases. For example, certain infections or medications cause similar reactions as above. There is even a disease named ‘drug- induced lupus’. Removing the triggering factor relieves the symptoms and is the best treatment in those cases.
The exact etiology of SLE is unknown but multiple genetic, environmental, and epigenetic factors are implicated. Infection is considered as an environmental factor that triggers the development or exacerbation of SLE in the genetically susceptible person. Also, some infectious diseases show similar features to SLE, making it difficult to distinguish between the infections and SLE. Since 1980’s when rheumatic features related with Human parvovirus B19 (HPV-B19) were first reported, the association between HPV-B19 infection and autoimmunity has been considerably studied.
Here, we describe a case of HPV-B19 infection in a female adult, which was misdiagnosed as SLE at initial presentation. The relevant literature is briefly reviewed.
Go to : 
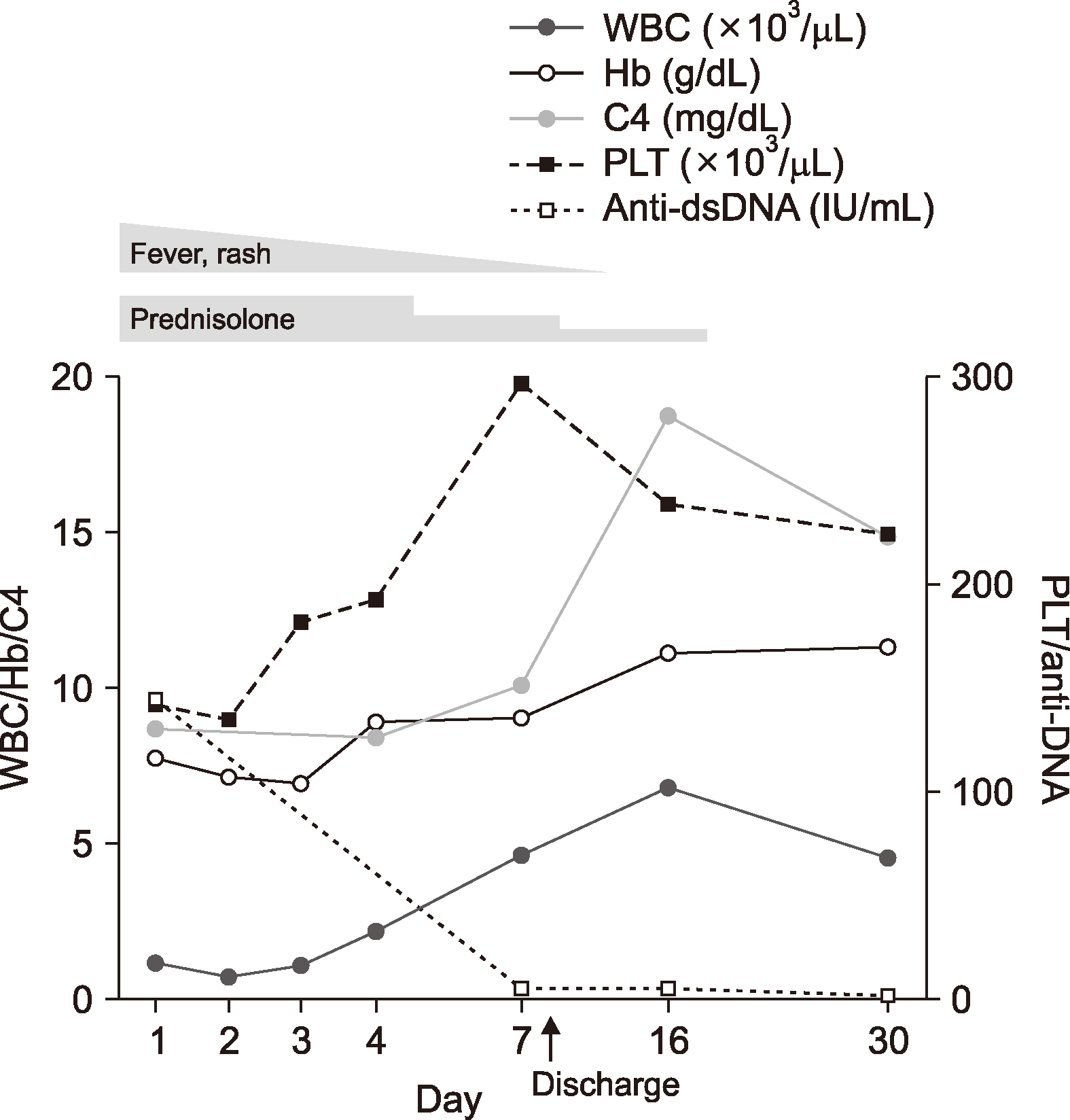
A previously healthy 46-year-old woman was admitted with one-week history of cough, fever, chilling, and skin rash of diffuse reticulated erythema on both lower legs and dorsum of feet. One day before admission, pain and swelling developed in multiple joints, including both knees, ankles, wrists and hands (proximal/distal interphalangeal joints). On admission, her body temperature was 37.7°C and blood test revealed pancytopenia, with a white blood cell count (WBC) of 1.240×103/μL, hemoglobin (Hb) of 7.7 g/dL, and platelet count (PLT) of 143× 103/μL. Serum creatinine and urine protein/creatinine (P/C) ratio were increased to 1.37 mg/dL and 0.7 mg/mg, respectively, showing proteinuria. Complements were decreased (C3: 41 mg/dL, C4: 8.1 mg/dL, total hemolytic complement: 17.4 U/mL). Both direct and indirect Coomb’s test were negative. Although it was not highly likely that the patient was affected by bacterial infection considering the normal levels of acute phase reactants (such as erythrocyte sedimentation rate, C-reactive protein, and ferritin), viral studies including Epstein-Barr (EBV), herpes simplex, varicella zoster, and HPV-B19 were also done assuming the symptoms such as cough and fever might be caused by viral respiratory infections. At the same time, autoantibodies were tested since overall findings until then indicated that SLE was the most suspicious diagnosis. Several autoantibodies were positive, including antinuclear antibody (ANA) (1:160, speckled), Anti-double stranded DNA antibody (anti-dsDNA Ab) (145.26 IU/mL; normally less than 7 IU/mL), and anti-proteinase 3-antineutrophil cytoplasmic Ab (PR3-ANCA, weakly positive as 1.68 IU/mL; normally less than 1.0 IU/mL). Anti-smith Ab, lupus anticoagulant, anti-cardiolipin Ab (Immunoglobulin G/Immunoglobulin M [IgG/IgM]) and anti-β2 glycoprotein 1 Ab were negative. Accordingly, SLE was highly suspicious, the patient was treated with methylprednisolone of 1 mg/kg/day (60 mg/day) from the first day of admission. Imaging studies such as abdominal computed tomography and kidney sonography were nonspecific. Skin biopsy from left calf was also nonspecific, showing mild superficial perivascular dermatitis. On the fifth day, polymerase chain reaction (PCR) detection of HPV-B19 in the blood was reported to be positive, confirming acute viral infection. IgM tests for other viruses were all negative except for EBV. Viral capsid antigen IgM/IgG, early antigens-diffuse IgM and nuclear antigen IgG were positive, which is seen in EBV reactivation. However, EBV-PCR was negative. Further, bone marrow biopsy was done to evaluate the cause of pancytopenia in suspicion of transient aplastic crisis accompanied by HPV-B19 infection. No abnormal cells were seen in bone marrow aspirates. Steroid was reduced to 30 mg/day on the sixth day, and cytopenia, proteinuria, arthralgia and skin rash gradually improved. On the seventh day of admission, pancytopenia was recovered with WBC of 4.650×103/μL, Hb of 9.0 g/dL, and PLT of 298×103/μL. Anti-dsDNA Ab was found to be negatively converted (Figure 1), and urine P/C ratio deceased to 0.14 mg/mg. The patient was discharged with 15 mg/day of prednisolone on the eighth day. One week after discharge, anti-dsDNA Ab was consistently negative, urine P/C ratio was decreased to 0.03 mg/mg, and complete blood count and complement levels were within normal limits. Steroid was discontinued at this point. After the next 2 weeks, laboratory findings were still within normal ranges with negative anti-dsDNA Ab. ANA was positive at the same titer. Follow-up was terminated after five months of disease onset, with the need to revisit in case of symptoms recurrence.
Go to : 
The present patient shows SLE-like syndrome associated with HPV-B19 infection. She presented with one week of fever, skin rash on lower extremities, arthralgia, and laboratory findings of proteinuria, leukopenia, positive ANA, anti-dsDNA Ab and hypocomplementemia. This satisfied both 2012 systemic lupus international collaborating clinics and 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for SLE at disease onset. However, all clinical and immunological abnormalities resolved within two weeks except ANA.
Lupus mimickers include infection, neoplasm, medication, vaccine and graft versus host disease. Infection is the most common etiology among them, and HPV-B19 takes the biggest part of infectious agents. According to a literature review study by Calixto et al. [2], HPV-19 was found in 88% of the patients with viral infections mimicking SLE. Other viruses such as EBV, cytomegalovirus, human immunodeficiency virus and hepatitis virus are also reported as lupus mimickers, but are rare compared with HPV-B19. In the present case, we consider HPV-B19 as the true pathogen. HPV-B19 can induce false-positive reaction through cross-reacting with EBV [3], which may explain the incoherent result of positive EBV-IgM observed with negative EBV-PCR. As well as SLE, HPV-B19 infection can mimic other autoimmune diseases, such as rheumatoid arthritis, vasculitis and systemic sclerosis. The present patient showed weak positivity for PR3- ANCA but did not reveal typical symptoms or signs of vasculitis except skin rash. According to the report by Hermann et al. [4], both PR3-ANCA and myeloperoxidase-ANCA Ab may occur transiently in patients with acute HPV-B19 infection, which turn negative within six months.
HPV-B19 is a single-stranded, human-pathogenic DNA virus. The viral genome encodes two structural capsid proteins (VP1 and VP2) and one nonstructural protein. The virus is transmitted mainly via respiratory route. Vertical or hematogenous transmission is also possible. Children are more likely to be infected than adults, and when the latter are infected, they tend to have more severe symptoms. Infection causes a biphasic clinical course. Flu-like symptoms may occur at first. During the second phase, slapped-cheek rash may develop in children followed by erythematous, maculopapular rash on trunk and extremities. Adults lack this slapped-cheek appearance. However, arthropathy is more common in adults, occurring in 60% of infected adults while appearing in 10% of children’s cases. Joints involvements are usually symmetric and non-destructive, begin in hands or knees, and occur twice as often in women as men. When a patient has underlying hemolytic disorder, transient aplastic crisis may occur. In immunosuppressed patients, aplastic anemia develops due to lack of neutralizing antibody production [5].
As seen in our case, clinical similarities between HPV-B19 infection and SLE exist such as skin rash, especially malar rash, arthritis, proteinuria, constitutional symptoms including fever, fatigue and myalgia. In a review of 28 cases of HPV-B19 associated SLE, arthropathy and malar rash were observed in 86% and 54% of the cases, respectively [6]. Immune complex has been postulated as possible mechanism. Although not seen in present patient, lymphadenopathy is also common in both diseases. Kikuchi’s disease, a benign disorder of lymph nodes in adults, is another lupus mimicker, and has to be ruled out if lymphadenopathy exists. There is a case report showing the relation between Kikuchi’s disease, SLE and HPV-B19, suggesting that Kikuchi’s disease may be a response to HPV-B19 infection in SLE [7]. In pediatric patients, Kawasaki disease along with SLE should be distinguished from HPV-B19 infection, since the HPV-B19 infection may induce similar clinical features of both diseases including lymphadenitis [8]. Laboratory similarities include hypocomplmentemia, elevated erythrocyte sedimentation rate, cytopenia (anemia, leukopenia, thrombocytopenia), positive Coomb’s test, and autoantibodies. About 25%∼68% of HPV-B19 infected patients show transient positive autoantibodies [9]. ANA is the most commonly found one whose positivity is up to 70%∼90% in some reports. Others include antiphos-pholipid Ab (anti-cardiolipin or anti-β2 glycoprotein 1), anti-dsDNA Ab, Anti-SSA/Ro Ab, anti-SSB/La Ab and rheumatoid factor. ANCA is uncommon although it is seen in our case. These autoantibodies generally resolve within three months, although persistent existence has also been reported [6]. However, there are also differences that may help to distinguish HPV-B19 from SLE (Table 1). Discoid rash, alopecia and Raynaud’s phenomenon are absent in HPV-B19 infection. Oral ulcer, serositis, organ involvements such as heart, kidney, spleen, eye, gastrointestinal tract (GI) and central nervous system (CNS) are rare [10]. Disease severity is usually mild and self-limiting. Chronic disease or persistent antibody production is uncommon. Anemia in HPV-B19 is usually secondary to bone-marrow suppression, whereas in SLE, it is due to chronic disease or autoimmune hemolysis [2].
Characteristics of HPV-B19 infection versus SLE
There are several proposed mechanisms in HPV-B19 induced autoimmunity. Molecular mimicry between host and viral proteins is one of the major pathogenic pathways. For example, anti-VP1 IgG from patients with chronic or recurrent arthritis, skin rash and specific IgM against HPV-B19 cross-reacts with keratin, collagen type II, single-stranded DNA and cardiolipin [11]. HPV-B19 binds to Ku80, a lupus autoantigen, activating the immune system [12]. Phospholipase activity of VP1-unique region triggers the production of anti-phospholipid Ab and contributes to inflammatory response induced by leukotrienes and prostaglandins [13]. Constant presence of viral antigen may induce nonspecific T-cell activation by acting as a superantigen.
HPV-B19 infection induced lupus-like syndromes in adults are rare, but have been reported in some case reports and series. However, to the best of our knowledge, they have not been reported in Korea yet. Only two articles on autoimmunity other than SLE in Korean patients were reported: one on rheumatoid arthritis [14], and the other on positive anti-SSA/Ro Ab [15]. Also, it is rare that EBV IgM or ANCA is positive in HPV-B19 infection induced lupus-like syndrome, making the diagnosis confusing. Although it is not common, possibility of HPV-B19 infections should be considered in adult patients with SLE features especially if the clinical and laboratory abnormalities resolve completely and rapidly.
Go to : 
HPV-B19 infections can present with clinical and laboratory features mimicking SLE at disease onset. To distinguish between the viral infection and the new-onset SLE, it may be helpful to see whether some characteristics of HPV-B19 infection, such as mild and self-limiting course, rarely involved heart/kidney/GI/CNS, absence of oral ulcer/alopecia/Raynaud’s phenomenon, and transient autoantibodies exist or not.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
M.Y.K. analyzed and interpreted the patient data and drafted the manuscript. J.J.L. analyzed and interpreted the patient data and reviewed and approved the final manuscript.
Go to : 
REFERENCES
1. Lisnevskaia L, Murphy G, Isenberg D. 2014; Systemic lupus erythematosus. Lancet. 384:1878–88. DOI: 10.1016/S0140-6736(14)60128-8. PMID: 31180031.


2. Calixto OJ, Franco JS, Anaya JM. 2014; Lupus mimickers. Autoimmun Rev. 13:865–72. DOI: 10.1016/j.autrev.2014.05.002. PMID: 24820523.


3. De Paschale M, Clerici P. 2012; Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol. 1:31–43. DOI: 10.5501/wjv.v1.i1.31. PMID: 24175209. PMCID: PMC3782265.



4. Hermann J, Demel U, Stünzner D, Daghofer E, Tilz G, Graninger W. 2005; Clinical interpretation of antineutrophil cytoplasmic antibodies: parvovirus B19 infection as a pitfall. Ann Rheum Dis. 64:641–3. DOI: 10.1136/ard.2004.024877. PMID: 15485998. PMCID: PMC1755429.


5. Broliden K, Tolfvenstam T, Norbeck O. 2006; Clinical aspects of parvovirus B19 infection. J Intern Med. 260:285–304. DOI: 10.1111/j.1365-2796.2006.01697.x. PMID: 16961667.


6. Sève P, Ferry T, Koenig M, Cathebras P, Rousset H, Broussolle C. 2005; Lupus-like presentation of parvovirus B19 infection. Semin Arthritis Rheum. 34:642–8. DOI: 10.1016/j.semarthrit.2004.07.008. PMID: 15692957.


7. Meyer O, Kahn MF, Grossin M, Ribard P, Belmatoug N, Morinet F, et al. 1991; Parvovirus B19 infection can induce histiocytic necrotizing lymphadenitis (Kikuchi's disease) associated with systemic lupus erythematosus. Lupus. 1:37–41. DOI: 10.1177/096120339100100107. PMID: 1845362.


8. Watanabe Y, Inoue Y, Takatani T, Arai H, Yasuda T. 2009; Self-limited lupus-like presentation of human parvovirus B19 infection in a 1-year-old girl. Pediatr Int. 51:411–2. DOI: 10.1111/j.1442-200X.2009.02829.x. PMID: 19500282.


9. Meyer O. 2003; Parvovirus B19 and autoimmune diseases. Joint Bone Spine. 70:6–11. DOI: 10.1016/S1297-319X(02)00004-0. PMID: 12639611.


10. Severin MC, Levy Y, Shoenfeld Y. 2003; Systemic lupus erythematosus and parvovirus B-19: casual coincidence or causative culprit? Clin Rev Allergy Immunol. 25:41–8. DOI: 10.1385/CRIAI:25:1:41. PMID: 12794260.


11. Lunardi C, Tiso M, Borgato L, Nanni L, Millo R, De Sandre G, et al. 1998; Chronic parvovirus B19 infection induces the production of anti-virus antibodies with autoantigen binding properties. Eur J Immunol. 28:936–48. DOI: 10.1002/(SICI)1521-4141(199803)28:03<936::AID-IMMU936>3.0.CO;2-X. PMID: 9541589.


12. Munakata Y, Saito-Ito T, Kumura-Ishii K, Huang J, Kodera T, Ishii T, et al. 2005; Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood. 106:3449–56. DOI: 10.1182/blood-2005-02-0536. PMID: 16076874.


13. Dorsch S, Liebisch G, Kaufmann B, von Landenberg P, Hoffmann JH, Drobnik W, et al. 2002; The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity. J Virol. 76:2014–8. DOI: 10.1128/JVI.76.4.2014-2018.2002. PMID: 11799199. PMCID: PMC135890.



14. Chun LH, Kim TY. 2001; Human parvovirus B19 and rheumatoid arthritis in Korea. J Korean Rheum Assoc. 8:14–9.
15. Yoon HJ, Lee SS, Juhng H. 2005; A case of parvovirus B19 related arthropathy associated with antinuclear antibody, anti-Ro/SSA and anti-La/SSB. J Korean Rheum Assoc. 12:52–6.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download