INTRODUCTION
Liver transplantation (LT) has become the treatment of choice for patients with end-stage liver disease because of improved results and broadening of indications. The shortage of organ donors and the increased demand for LT have led to initiatives to increase the availability of liver grafts for LT. The acceptance of old and marginal liver donors, along with the development of alternative techniques including liver graft splitting, living donors, and domino procedures, have been proposed to lower the mortality rate of patients on the waiting list. Although these procedures have helped increase the available donor organ pool, a profound shortage still remains.
Even though the organs have been exposed to periods of warm ischemic injury, interest in utilizing organs from non–heart-beating donors (NHBDs) has increased because of the shortage of organs and reports of good graft function and graft survival [
1-
6]. LT from NHBDs has been scarcely performed in Korea because only Maastricht category IV (cardiac arrest in a brain-dead donor) is legally permitted. To the best of our knowledge, only three Korean cases of LT from NHBDs have been reported in the literature [
7-
9]. Herein, we present one case of LT from an NHBD with extracorporeal membrane oxygenation (ECMO) support performed at a high-volume LT center in Korea.
Go to :

CASE REPORT
This study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-0797), and the requirement for informed consent was waived.
The case was a 42-year-old male patient who had been diagnosed with alcoholic liver cirrhosis. The laboratory findings at admission were serum total bilirubin 14.1 mg/dL, serum creatinine 1.2 mg/dL and prothrombin time international normalized ratio 2.3, thus making the model for end-stage liver disease score 28 (
Fig. 1A). This patient had alert mental status with moderate ascites, with Child-Turcotte-Pugh score of 11. He had a past history of heavy alcohol drinking for 20 years. His sister was evaluated for liver donation, but excluded from donation because of unacceptable left-right liver volume proportion. Thus, he was enrolled to the waiting list of the Korean Network for Organ Sharing (KONOS) with old status 2B.
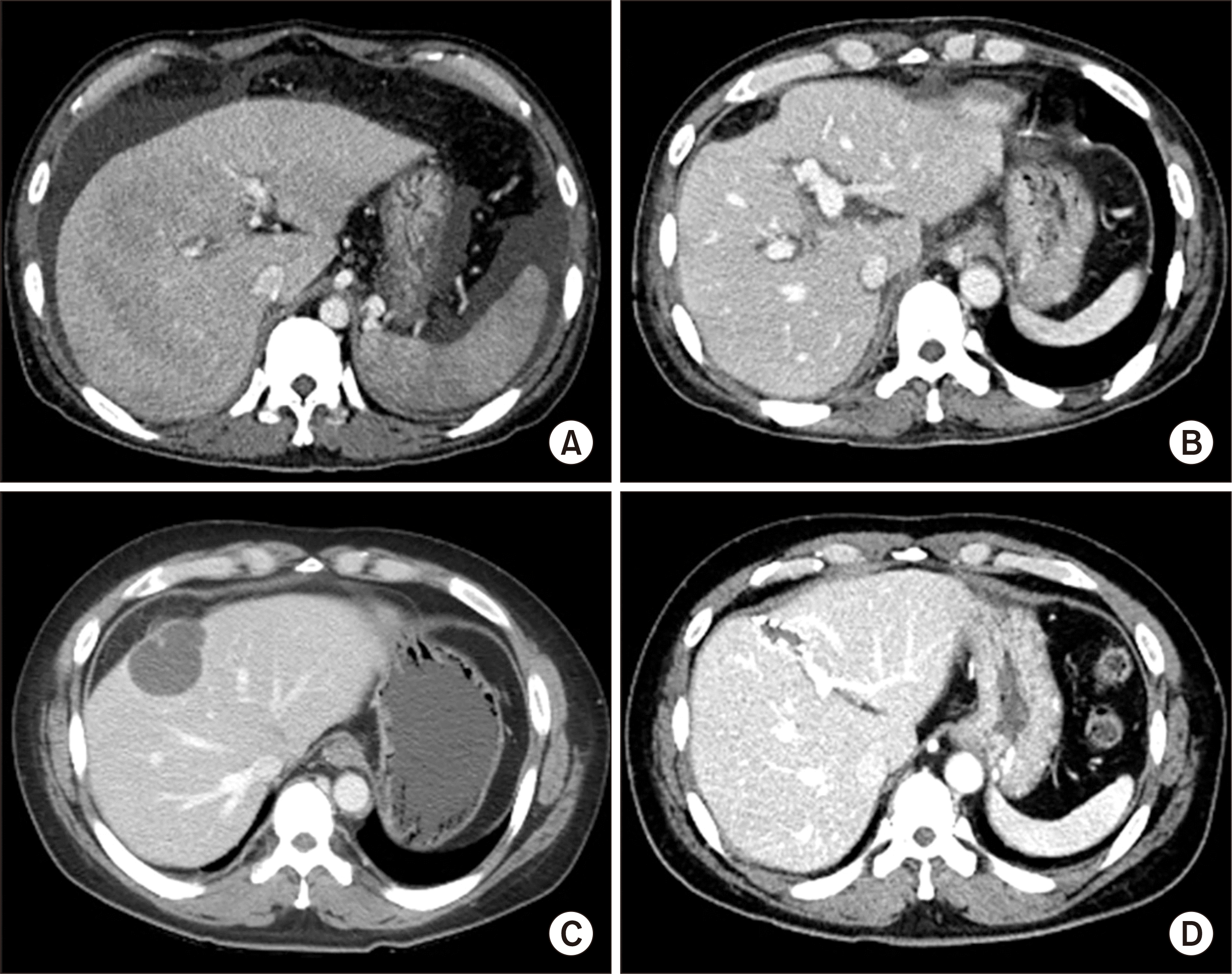 | Fig. 1Computed tomography (CT) findings. (A) Pretransplant CT scan shows overt liver cirrhosis. (B) CT scan taken at 1 week after transplantation shows no significant abnormality. (C) CT scan taken at 2 years after transplantation shows a cystic mass in the central part of the liver. (D) CT scan taken at 8 years after transplantation shows no abnormal findings. 
|
After a waiting period for 3 weeks, this patient was allocated to a 47-year-old female donor. She had been diagnosed with diabetes mellitus, hypertension, congestive heart failure and atrial fibrillation. Cerebral infarct developed at home and the patient was transferred to a nearby hospital and diagnosed with potential brain death (
Fig. 2). She was transferred to our institution for organ donation. She experienced two episodes of cardiopulmonary resuscitation (CPR) for cardiac arrest before transfer to our institution. She also experienced two sessions of ventricular fibrillation in our institution. Thus, CPR was preformed repeatedly, and showed return of spontaneous circulation. She was diagnosed of being brain-dead during the evaluation process for brain death, and her family members agreed to organ donation.
 | Fig. 2Computed tomography findings of the donor’s brain. It was taken for the initial diagnosis of intracranial hemorrhage. 
|
During the next-step evaluation process for the second confirmation of brain death, the blood pressure of this patient dropped progressively and was not responsive to inotropic agents. Thus, venous-arterial type ECMO was started through cannulation of the right femoral vein and artery. After starting ECMO, the patient’s initial blood pressure was 81/64 mmHg with a mean value of 69 mmHg, but the systolic blood was gradually decreasing. The patient was urgently moved to the operating room for organ recovery. At that time, there was nearly no cardiac rhythm at the electrocardiogram monitor. In order to confirm loss of the heart beats, ECMO was stopped. The total running time of ECMO was 317 minutes. Just after ECMO stop, no arterial pulsation and cardiac rhythm were detected at the vital sign monitor, thus asystole was declared. After additional waiting for 5 minutes, cardiac death was declared, and then heparin 20,000 units was administered. Immediately after the declaration of death, a skin incision was initiated and the mesenteric root was rapidly mobilized. Just after insertion of an aorta perfusion cannula, histidine-tryptophan-ketoglutarate (HTK) solution was perfused with aorta cross-clamping. It took 6 minutes from skin incision to aorta perfusion (
Fig. 3).
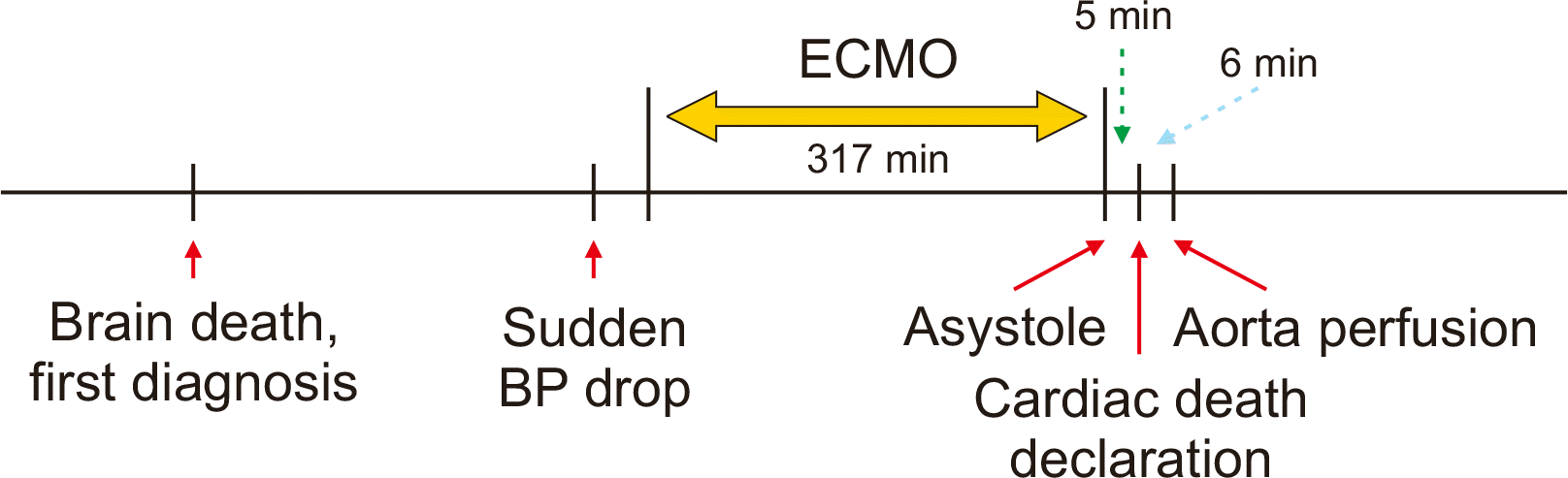 | Fig. 3The time sequence of process for donation after cardiac death. BP, blood pressure; ECMO, extracorporeal membrane oxygenation. 
|
After aorta perfusion with HTK solution, the liver appeared grossly normal and a frozen-section liver biopsy showed only mild portal inflammation with no fatty change. Thus, we decided to use the liver graft. The liver and two kidneys were harvested rapidly with minimal dissection. Additional portal vein perfusion was performed at the back table to wash out the residual blood within the liver graft. The liver graft weighed 1,460 g, yielding a graft-recipient weight ratio of 2.16%.
The recipient hepatectomy and liver graft implantation were performed according to the standard procedures of adult whole LT. Classical interposition anastomosis of the retrohepatic inferior vena cava was performed under active venovenous bypass. End-to-end anastomosis of the portal vein was performed and reperfusion started. The cold ischemic time was prolonged to 443 minutes because the recipient operation was not well synchronized with the urgent donor operation, and the warm ischemic time until portal reperfusion was 33 minutes. Thereafter, hepatic artery anastomosis using a branch patch was performed. Finally, biliary reconstruction was done through duct-to-duct anastomosis with insertion of a T-tube. The explant liver pathology showed macronodular cirrhosis, which was compatible with that of alcoholic liver cirrhosis (
Fig. 4).
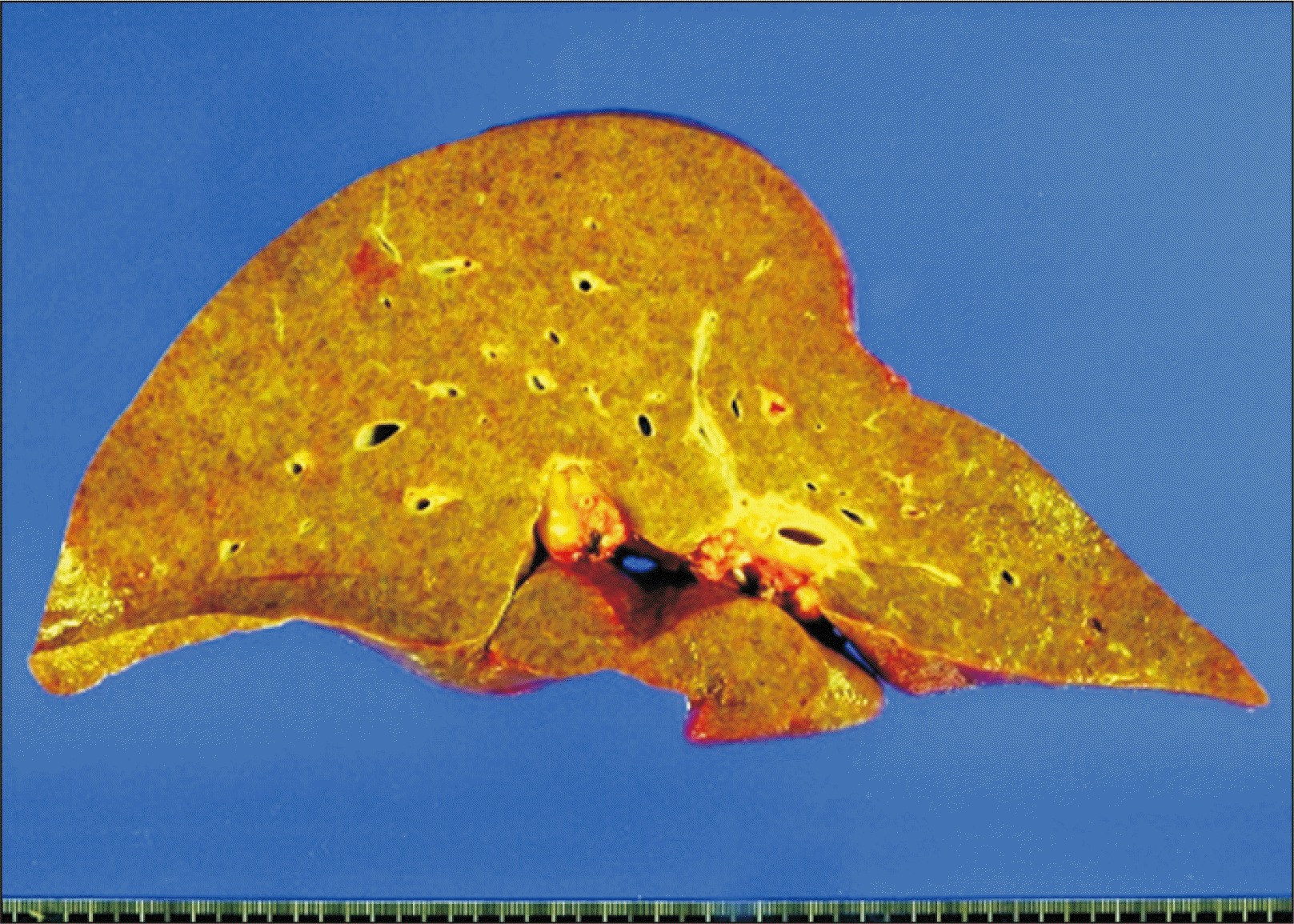 | Fig. 4Gross photograph of the explanted liver. There existed macronodular liver cirrhosis compatible with alcoholic liver cirrhosis. 
|
The patient recovered from the LT operation uneventfully with peak aspartate transaminase level of 1,835 IU/L and alanine transaminase level of 656 IU/L during the first posttransplant week and was discharged 16 days after LT (
Fig. 1B). Two years after LT, a liver mass was detected (
Fig. 1C), and a percutaneous biopsy suggested idiopathic posttransplant hepatitis. The mass was resected through a laparotomy and pathologically proven to be chronic granulomatous inflammation with necrosis. The patient has been doing well for 9 years after LT (
Fig. 1D).
Go to :

DISCUSSION
Brain-dead patients undergo deterioration in the metabolic function of other organs before the determination of brain death and 80% of them experience cardiac death within 120 hours because cardiopulmonary function deteriorates despite active treatment [
2]. Metabolic disorders and hematologic deterioration cause cardiac arrest. It is important to maintain hematologic stability and physiological balance in the management of deceased organ donors.
If a potential donor cannot maintain sufficient blood pressure before the diagnosis of brain death, donation after cardiac death (DCD) can be taken into account. NHBDs, who are declared dead due to cardiopulmonary function effacement are classified into four categories according to the Maastricht criteria determined at the Maastricht Workshop [
10]. In uncontrolled NHBD, which includes category I and II, category I refers to patient dead on arrival, whereas category II refers to death due to unsuccessful CPR. In controlled NHBD, which includes category III and IV, category III means the status of readiness of organ retrieval from a donor who is not brain-dead and whose life support system is withdrawn with the consent of the patient’s family for organ donation. Immediately after cardiopulmonary function ceases when the administration of medicine and respiratory aid to the donor are terminated, the waiting surgical team recovers the organs. Category IV refers to cardiac arrest in a brain-dead donor [
10,
11]. This category includes patients who experience an unexpected cardiac arrest after a diagnosis of brain death and during donor management but prior to the planned organ recovery. In patients in this category, it is likely that health-care professionals will first try to restore the adequate circulation and perfusion of organs, but when unsuccessful, the patient can be considered for DCD. The NHBD in the present case was classified into category IV. If the blood pressure of this donor had been maintained, she would be a brain-dead donor because she had been diagnosed with brain death once and the second final confirmatory diagnosis was yet to be made.
Over the last 10 years, with the increasing use of DCD donors, Maastricht criteria, including Modified Maastricht Classification for DCD (Madrid 2011;
http://www.ont.es/infesp/Paginas/DocumentosdeConsenso.aspx), Modified Maastricht classification for DCD (2012) [
12], and Modified Maastricht Classification for DCD (Paris 2013) [
11], have been produced by the expert European working groups. According to the Modified Maastricht Classification for DCD (Paris 2013), category IV is defined as “cardiac arrest while life-brain dead” and “sudden cardiac arrest after brain death diagnosis during donor life-management but prior to planned organ recovery”.
For heart-beating donors, a warm ischemic injury occurring between the time of cardiac arrest and the onset of perfusate perfusion is usually absent because the cessation of ventilator support and cardiac arrest occur shortly before cross-clamping of the aorta and perfusate perfusion. For NHBDs, the degree of warm ischemic injury varies case by case, so the postoperative results of LT fluctuate [
3]. Since the liver is vulnerable to ischemic injury, unlike the kidney, graft dysfunction or non-function is a matter of concern in LT using NHBDs, primarily because a warm ischemic injury is inevitable. To increase the graft survival rate, the warm ischemic time should be within 30 minutes, the donor age should be ≤40 years, and the cold ischemic time should be <8 hours [
6,
13]. A study reported that the engraftment failure rate increased to 60% when the cold ischemic time was >12 hours, whereas the engraftment failure rate decreased to 10% or below in the case of cold ischemic time of ≤8 hours [
14]. Various attempts have been made, including cardiopulmonary bypass, ECMO, and super rapid retrieval, to reduce warm ischemic injury in NHBDs [
5]. In controlled NHBDs, like this case, the actual warm ischemic injury was not significantly prolonged compared to heart-beating brain-dead donors because cardiopulmonary support continued until several minutes (sometimes more than 10 minutes) before the start of the skin incision.
In the Korean setting regarding NHBDs, only NHBDs of Maastricht category IV are permitted for organ donation by law. This regulation has hindered the increased use of NHBDs. In reality, there were some attempts to use NHBDs 10 years ago, but not in recent years. The Korean Society for Transplantation intends to promote organ transplantation using NHBDs primarily because of the persistence of profound organ shortage. The annual number of deceased organ donors temporarily exceeded 10 per million in 2016 [
15], but has since decreased. The annual number of deceased organ donors and those undergoing deceased donor LT in Korea were 573 and 508 in 2016, 515 and 450 in 2017, 449 and 369 in 2018, and 450 and 391 in 2019, respectively. This trend might be associated with certain social issues including the Life Insurance Decision Act regulating the termination of life-sustaining treatment. According to this law, the termination of life support for patients in Maastricht category III appears to be permissible for the patient’s death, but not for organ donation.
To the best of our knowledge, only three Korean cases of LT from NHBDs have been reported to date in the literature. The first Korean case was LT with a liver originating from Maastricht category 4 NHBD. The donor was moved to the operation room after three times of CPR. Cardiac arrest occurred 15 minutes after stopping ventilation. After a waiting time for 5 minutes, a skin incision was performed. Super-rapid technique was used for the donor procedure. The recipient was discharged at 56 days after LT [
7]. Another case was a report focused on donor anesthesia and thus, lacked the posttransplant outcome [
8]. The other was a case of triple LTs, in which the second LT used an NHBD and the graft experienced primary non-function [
9].
In conclusion, NHBDs must be used as a method to increase the potential pool of organ donors, but it is very limited in the current Korean setting. The promotion of DCD requires changes toward enhanced public awareness and acceptance of donating organs, and legal support at the government level.
Go to :






 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download