INTRODUCTION
Drug-induced liver injury (DILI) is an important liver disease with a worldwide incidence of 14 to 19 cases per 100,000 persons [
1,
2]. Approximately 3%–5% of hospital admissions for jaundice and 10% of acute hepatitis cases are associated with DILI [
3]. DILI is the most common cause of acute liver failure (ALF) in Western society [
4,
5]. Azithromycin, a semisynthetic macrolides derived from erythromycin, was approved in 1994 [
6]. It is extensively prescribed for treating otitis media, upper respiratory tract infections, bronchitis, and community-acquired pneumonia [
7]. Azithromycin is frequently selected to first line antibiotics in patients with respiratory infection in Korea. The prescription rate of macrolide including azithromycin was higher (46.8%–49.0%) than that of penicillin (2.1%–2.5%) or third-generation cephalosporin (18.2%–19.5%) during 2011–2015 [
8]. Common adverse effects of azithromycin include nausea, vomiting, abdominal pain and diarrhea [
9].
Azithromycin-induced liver failure needed to liver transplantation has rarely been reported. In the United States, a 60-year-old woman with underlying nonalcoholic steatohepatitis was treated for a respiratory tract infection with azithromycin for 3 days and developed fatigue, anorexia and nausea 4 days later, followed by jaundice and deterioration of mental status. She underwent liver transplantation 1 month after the initial presentation, and recovered from progressive liver failure [
10]. Herein, we report a first case of liver transplantation for azithromycin-induced liver failure in a patient without previous liver disease in Korea.
CASE REPORT
This study was approved by the Institutional Review Board of Kosin University Gospel Hospital (IRB No. 2020-05-014). Informed consent was waived by the Review Board due the retrospective case report. This study was conducted in compliance with the principles of the Declaration of Helsinki.
A 68-year-old woman with jaundice was admitted to our hospital. Jaundice had developed 3 days before the visit. She manifested general weakness, fatigue, and decreased appetite. Although icteric sclera was observed, no other abnormal findings were noted on physical examination. On hospitalization, all her vital signs were normal range. Computed tomography (CT) and bronchoscopy revealed acute bronchitis, and she was administered tranexamic acid (250 mg), N-acetylcysteine (200 mg), dihydrocodeine, and azithromycin (250 mg) for a week. She had no history of allergic diseases and denied alcohol consumption and smoking.
Initial laboratory test results were as follows: white blood cell count, 9,100/mm
3; hemoglobin level, 13.7 g/dL; platelet count, 149,000/mm
3; aspartate aminotransferase level, 1,631 IU/L; alanine aminotransferase (ALT) level, 1,711 IU/L; total bilirubin level, 24.20 mg/dL; direct bilirubin level, 14.99 mg/dL; alkaline phosphatase (ALP) level, 226 IU/L; gamma-glutamyl transferase level, 232 U/L; total protein concentration, 5.3 g/dL; albumin level, 3.1 g/dL; blood urea nitrogen level, 6.4 mg/dL; creatinine level, 0.5 mg/dL; sodium concentration, 142 mmol/L; potassium concentration, 3.7 mmol/L; amylase level, 29 IU/L; lipase level, 53 IU/L; and ammonia concentration, 163 μMol/L. Prothrombin time was extended to 37.5 seconds and partial thromboplastin time was extended to 55.9 seconds. The high sensitivity C-reactive protein level was 0.932 mg/dL.
Fig. 1 summarizes the time course of blood tests for liver function. Hepatitis A virus immunoglobulin M (IgM) antibody, hepatitis B surface antigen, anti-HCV, Epstein-Barr virus (EBV) viral capsid antigen-IgM, EBV early antigen-diffuse restrict IgM, cytomegalovirus (CMV) IgM, and CMV real-time polymerase chain reaction tests were all negative. Herpes simplex virus (HSV)-IgM (enzyme immunoassay [ELISA]) was positive at 1.4 and HSV-immunoglobulin G (IgG; ELISA) was also positive at 23.5. Toxoplasma IgM and human immunodeficiency virus serum tests were found to be negative. In addition, antinuclear antibody, anti-mitochondrial antibody, anti-smooth muscle antibody and liver kidney microsomal antibody tests were all negative. The IgG serum level was normal at 774.6 mg/dL.
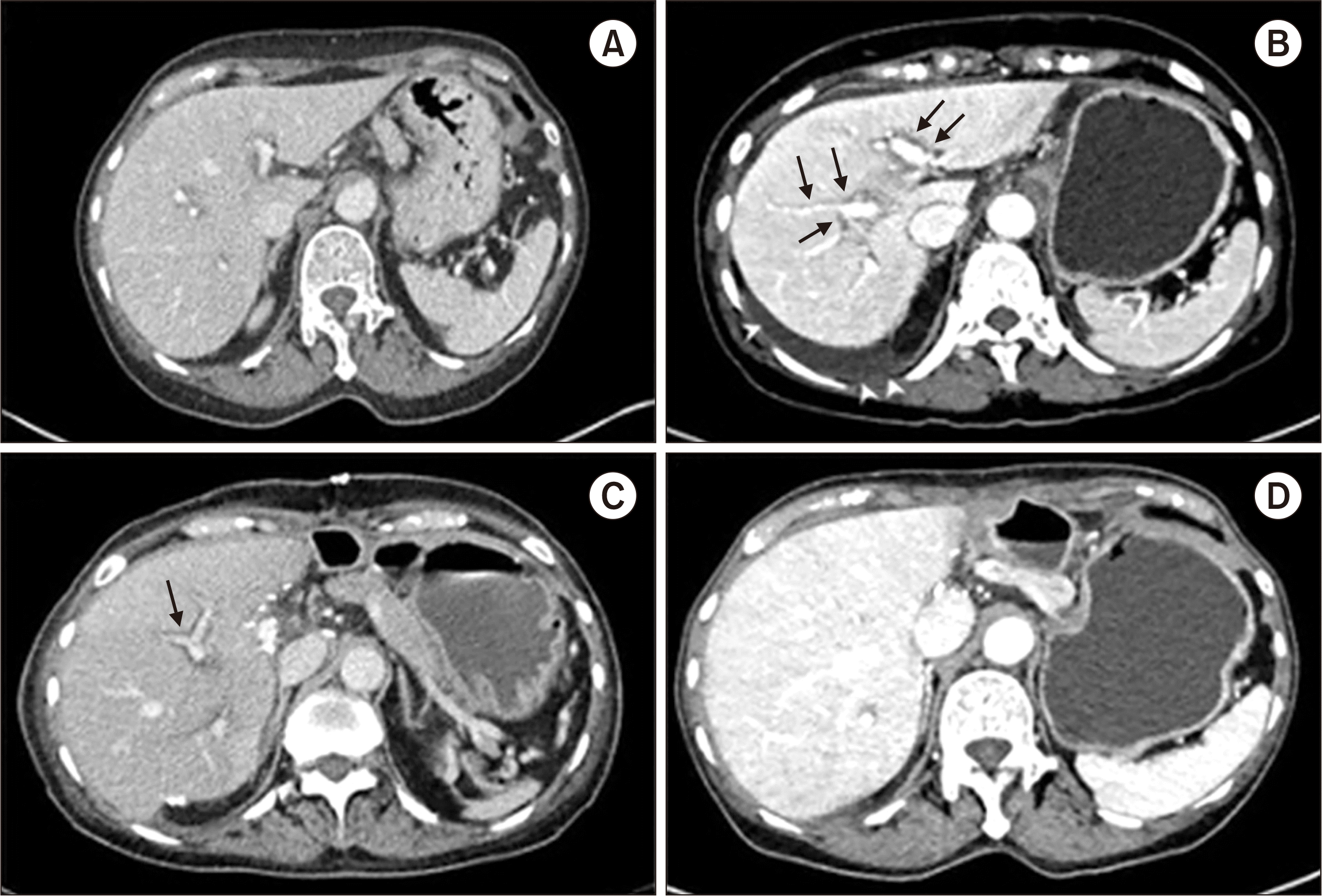
The CT image obtained of the liver in the week before initiating azithromycin treatment showed normal findings (
Fig. 2A). However, on the day of admission, periportal edema, gallbladder wall edema, and small amount of ascites were observed in the abdominal CT scan (
Fig. 2B).
She was clinically suspected of having azithromycin-induced liver injury and all medications were discontinued. The Roussel Uclaf Causality Assessment Method (RUCAM) scores for tranexamic acid, N-acetylcysteine, and dihydrocodeine were all 4 (possible), and the RUCAM score for azithromycin was 7 (probable) (
Table 1) [
11]. The R value for phenotype assessment was more than 5 and was considered to be a hepatocellular type [
10].The diagnosis of azithromycin-induced liver injury and ALF was made. The patient received medical treatment. However, laboratory test values had worsened (
Fig. 1). On the 3rd day of hospitalization, flapping tremor was observed, and the level of consciousness decreased to drowsiness. Hepatic encephalopathy persisted and the consciousness deteriorated to semi-coma. On the 8th day, she underwent emergency living donor liver transplantation, receiving a 950 g right lobe liver graft from a 35-year-old male donor, the patient’s son. Gradual recovery of consciousness and liver function was observed after the transplant. The patient was discharged from the hospital after 20 days of transplant. Currently, she is alive, asymptomatic, and with adequate liver graft function after 25 months of transplant (
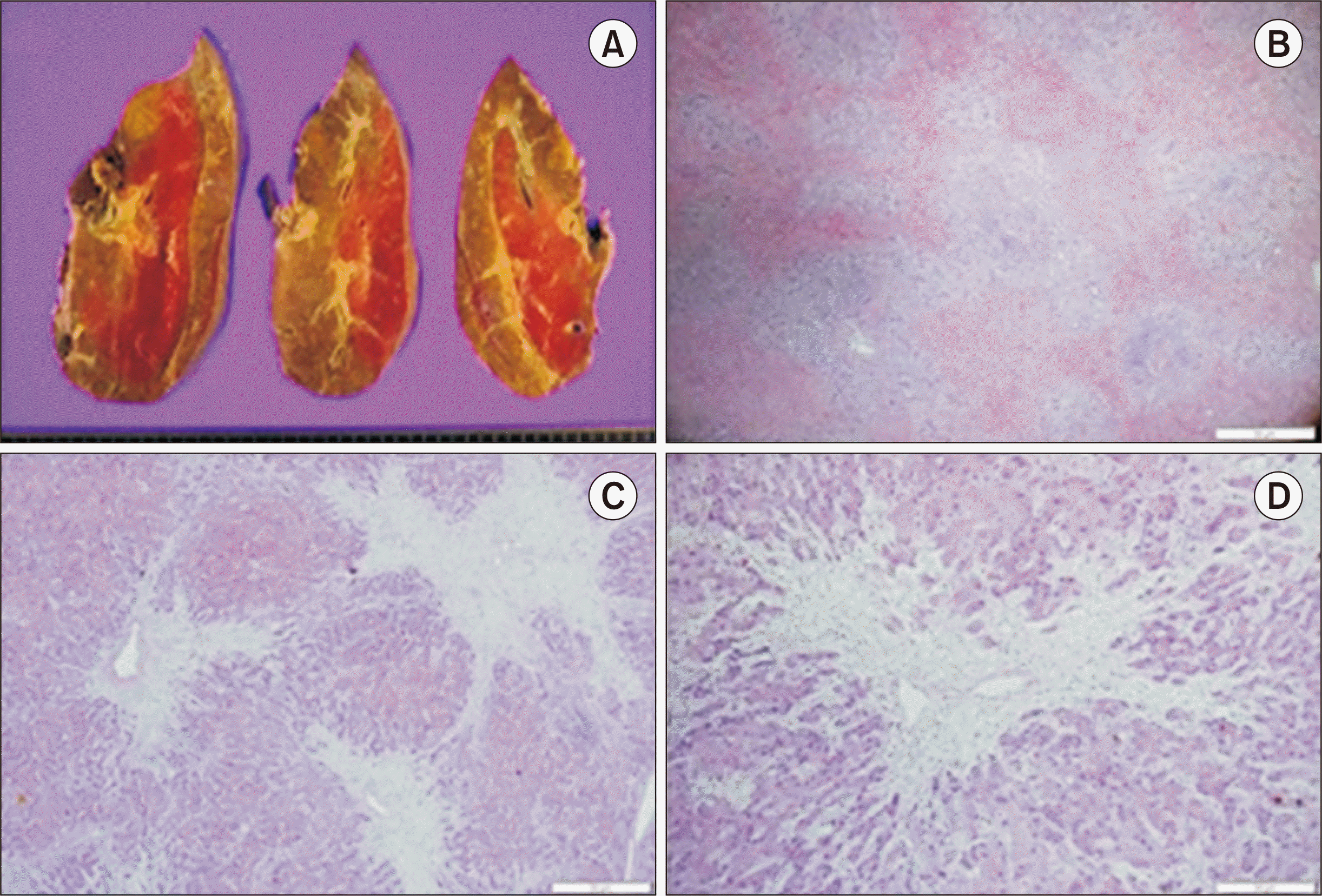
Fig. 2C and D). Histological examination of the explanted liver revealed fulminant hepatitis, zone 3 necrosis, and extensive hepatocyte death (
Fig. 3).
DISCUSSION
DILI is typically classified as direct or idiosyncratic. Direct hepatotoxicity is common, predictable, and dose–dependent. However, idiosyncratic hepatotoxicity can rarely occur due to agents with little or no intrinsic toxicity. It develops independently of drug route or duration of administration or drug dose [
12]. Azithromycin, a culprit drug in this study, induced hepatotoxicity that is typically classified to idiosyncratic mechanism [
10].
Based on the R value, idiosyncratic hepatotoxicity is classified into three phenotypes: hepatocellular (R ≥5), cholestatic (R ≤2), and mixed (2≤ R ≤5) [
13]. The R value is the ratio of ALT to ALP relative to their respective normal upper limits—[serum (AST/ALT the upper limit of normal [ULN])÷(ALP/ALP ULN)] [
10]. Hepatocellular phenotype is the most common and is characterized by a marked increase in ALT and a slight increase in ALP [
12,
14]. According to Hy's law by Hyman J. Zimmerman, the mortality rate of cholestatic phenotype with jaundice is low; however, the mortality rate of hepatocellular phenotype with jaundice is high, usually 10% or higher [
15]. We reported a case of woman without previous liver disease who developed azithromycin-induced ALF. This case represents a fatal clinical course with icteric hepatocellular injury.
Azithromycin-induced idiosyncratic hepatotoxicity is predominantly present as cholestatic or mixed phenotype. It has been reported that most of the patients exhibit benign clinical features, rarely patients exhibit fatal or chronic liver injury; however, the exact mechanism of injury is unknown [
10]. Herein, we reviewed 22 cases of azithromycin-induced liver injury that were previously reported and classified according to the three phenotypes as follows: hepatocellular, 10 patients (45.5%); cholestatic, 8 patients (36.3%); and mixed, 4 patients (18.2%) (
Table 1). The median (range of quartiles) age of all the patients was 46 years (19.8–66), and the proportion of women was 77.3%. In our case, the patient was a 68-year-old woman, older than the median age of all the aforementioned patients as well as the hepatocellular group (53.5 years [36.8–60]). Jaundice developed 4 days after azithromycin prescription, faster than the median (range of quartiles) days of all the cases (17.5 days [8.5–30.8]) as well as the hepatocellular group (20.5 days [15.3–32.5]). The median (range of quartiles) peak serum levels of ALT and ALP in the hepatocellular group were 1,271.5 IU/L (923.5–3,201.3) and 224.5 IU/L (159.5–444), respectively. In our case, peak ALT and ALP levels were 1,711 IU/L and 226 IU/L, respectively, close to those in the hepatocellular group. The median (interquartile range) peak total bilirubin level in the hepatocellular group was 17 mg/dL (10.8–21.5), whereas in our case it was 29.67 mg/dL. As described earlier, it is thought to be highly related to the fatal clinical course of liver transplantation. Pathologic findings were described in 11 of the 22 patients. Of the 11 patients, four cases showed hepatitis with no visible cholestasis, two cases showed acute cholestasis. Three other cases reported cholestatic hepatitis. Of the remaining two cases, one showed zone 3 necrosis and one showed complex findings. A total of 13 (59.1%) of 19 patients available for follow-up fully recovered, and the recovery period was 4–60 days after drug discontinuation. Four patients (18.2%) showed chronic progression. One patient (4.5%) recovered after liver transplantation, one patient (4.5%) died. To summarize, the latency in our case was much shorter than that of previous cases, and our case patient did not have underlying liver disease compared to patients with previous cases.
In conclusion, we report a case of azithromycin-induced liver failure treated liver transplantation in the patient without underlying liver diseases in Korea. Because azithromycin-induced liver injury is unpredictable, it is important to suspect DILI when a patient manifests acute jaundice and abdominal pain after administering azithromycin.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download