INTRODUCTION
Liver transplantation (LT) is an effective treatment for patients with end-stage liver disease because of improved results and broadening of indications. For pediatric patients, LT with size-matched whole liver allografts from pediatric donors is considered as ideal due to lower complication rates and better survival compared with other variant types of LT [
1]. However, the number of liver allografts recovered from pediatric donors is very limited in Korea. Furthermore, the body size of pediatric patients is widely variable from infant to adolescent, thus donor-recipient body size matching is much more complex compared with adult-to-adult deceased donor liver transplantation (DDLT).
Because of low incidence of pediatric deceased donors and complex donor-recipient body size matching, split LT and living donor LT have been more frequently performed for pediatric patients than LT with whole liver grafts [
2]. Small-sized whole liver grafts from young pediatric donors have usually been allocated to young pediatric recipients, and liver grafts from adolescent donors have been allocated to both adolescent and adult recipients, and those from adult donors have also been reciprocally allocated to adolescent recipients [
2-
6]. There exists only limited detailed information on pediatric DDLT in Korea, thus, there is an essential need to analyze the status of pediatric DDLT using whole liver grafts [
2,
6]. We herein investigated the incidence and outcomes of pediatric DDLT using whole liver grafts in a high-volume LT center.
Go to :

DISCUSSION
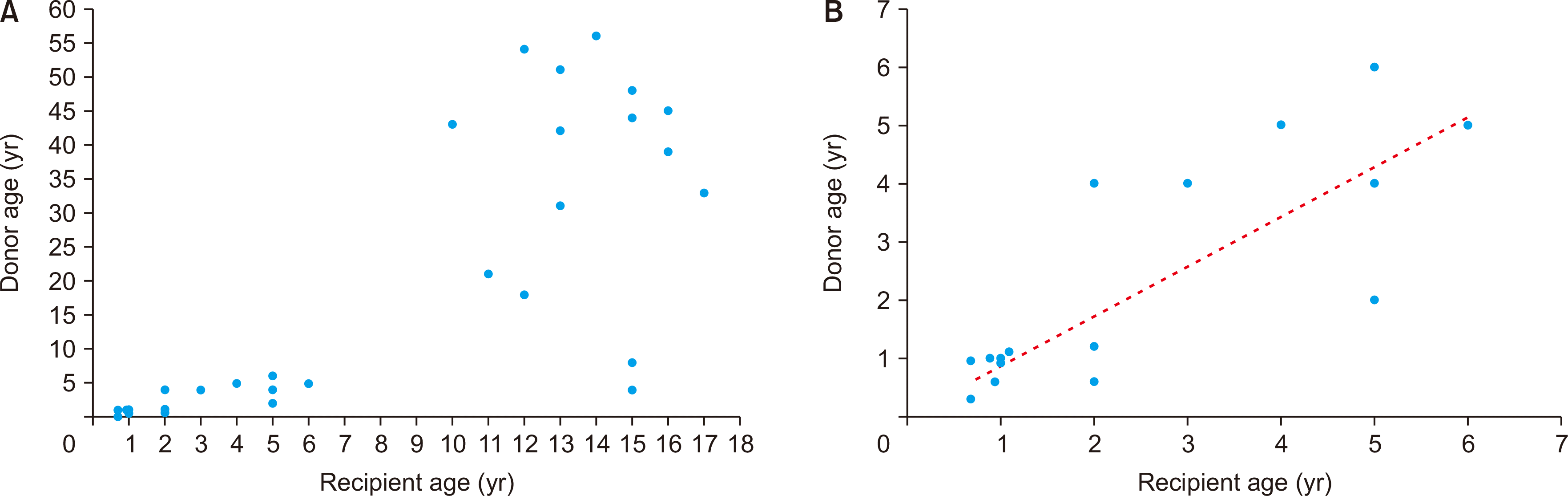
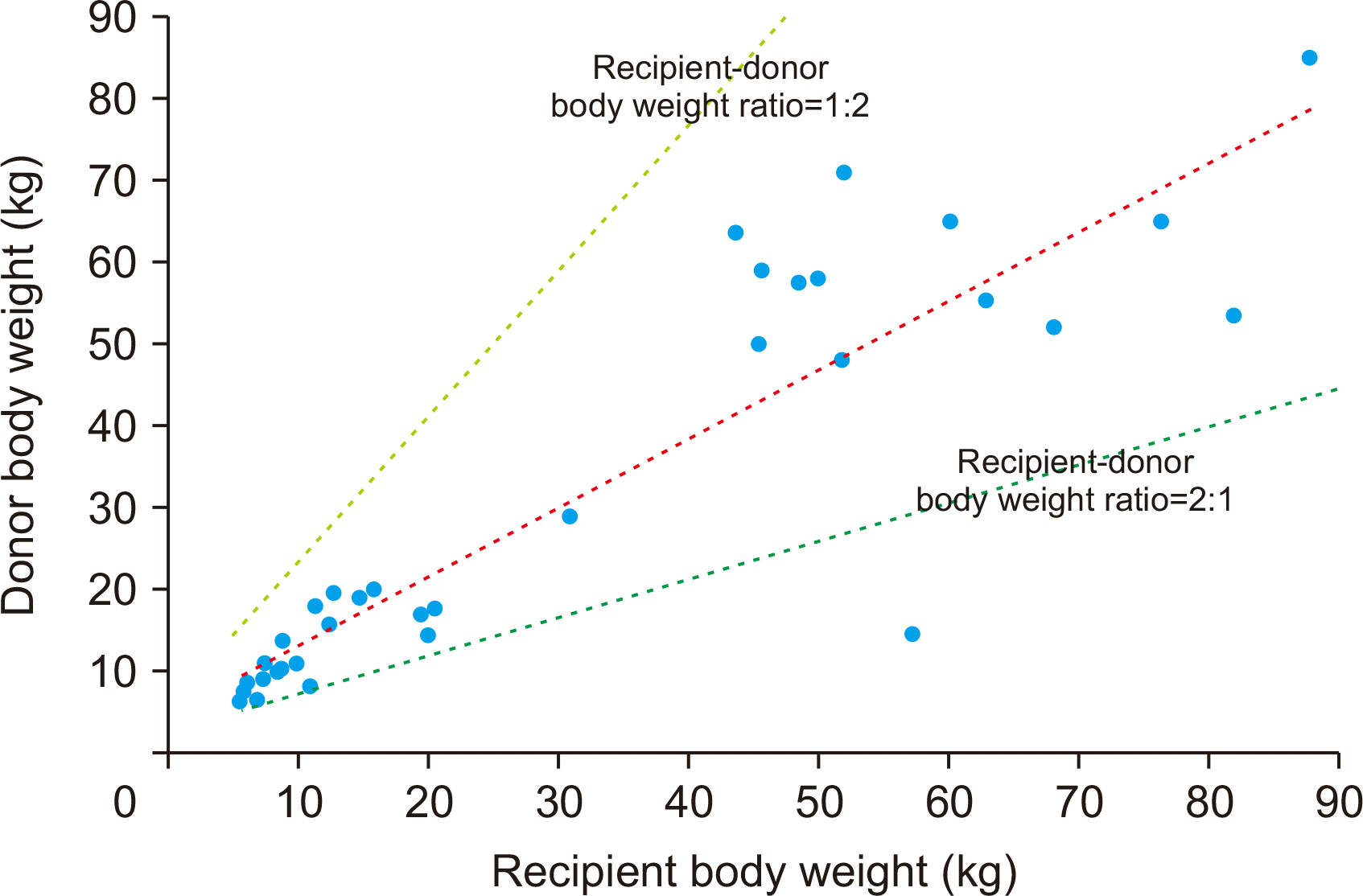
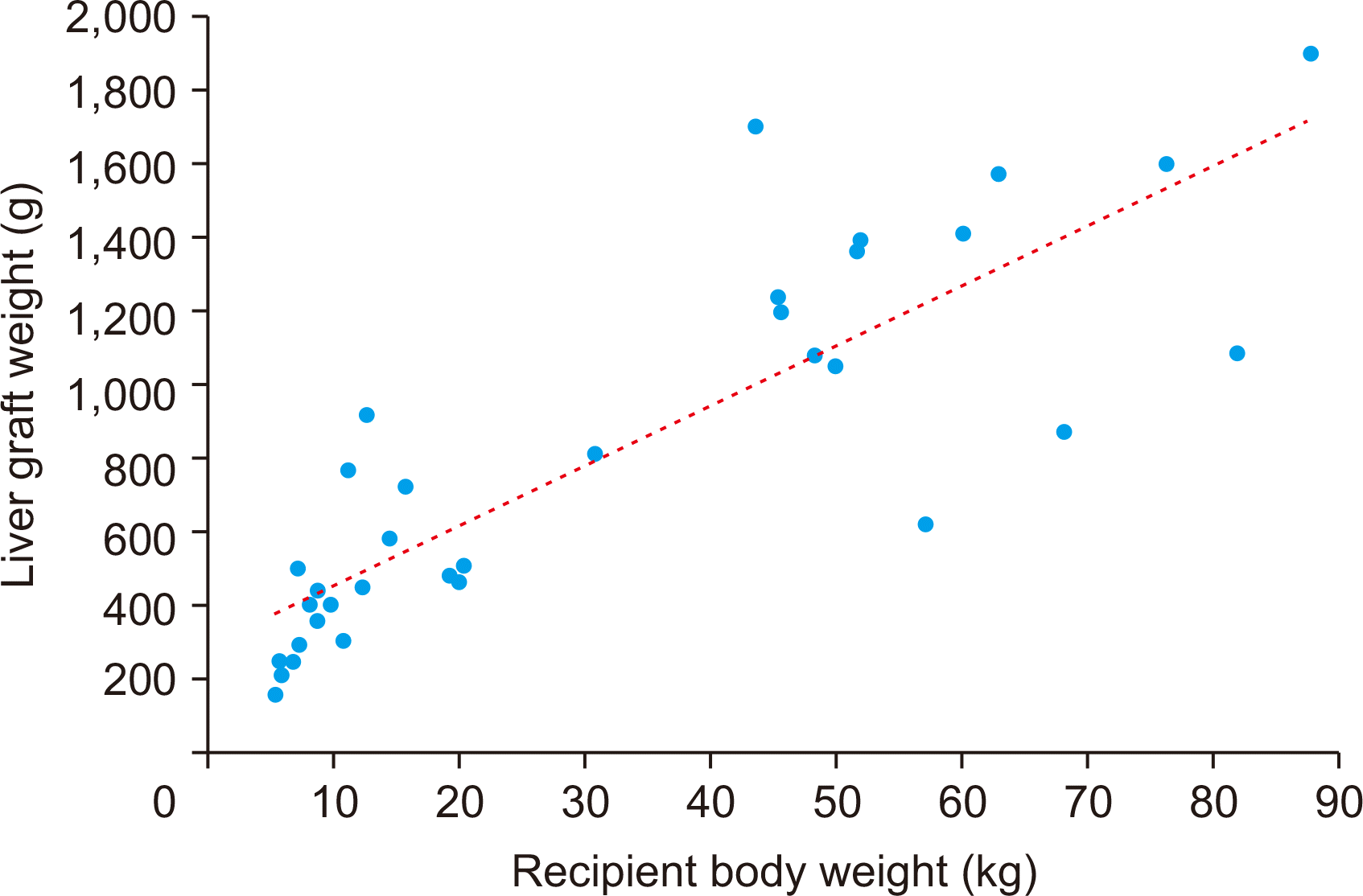
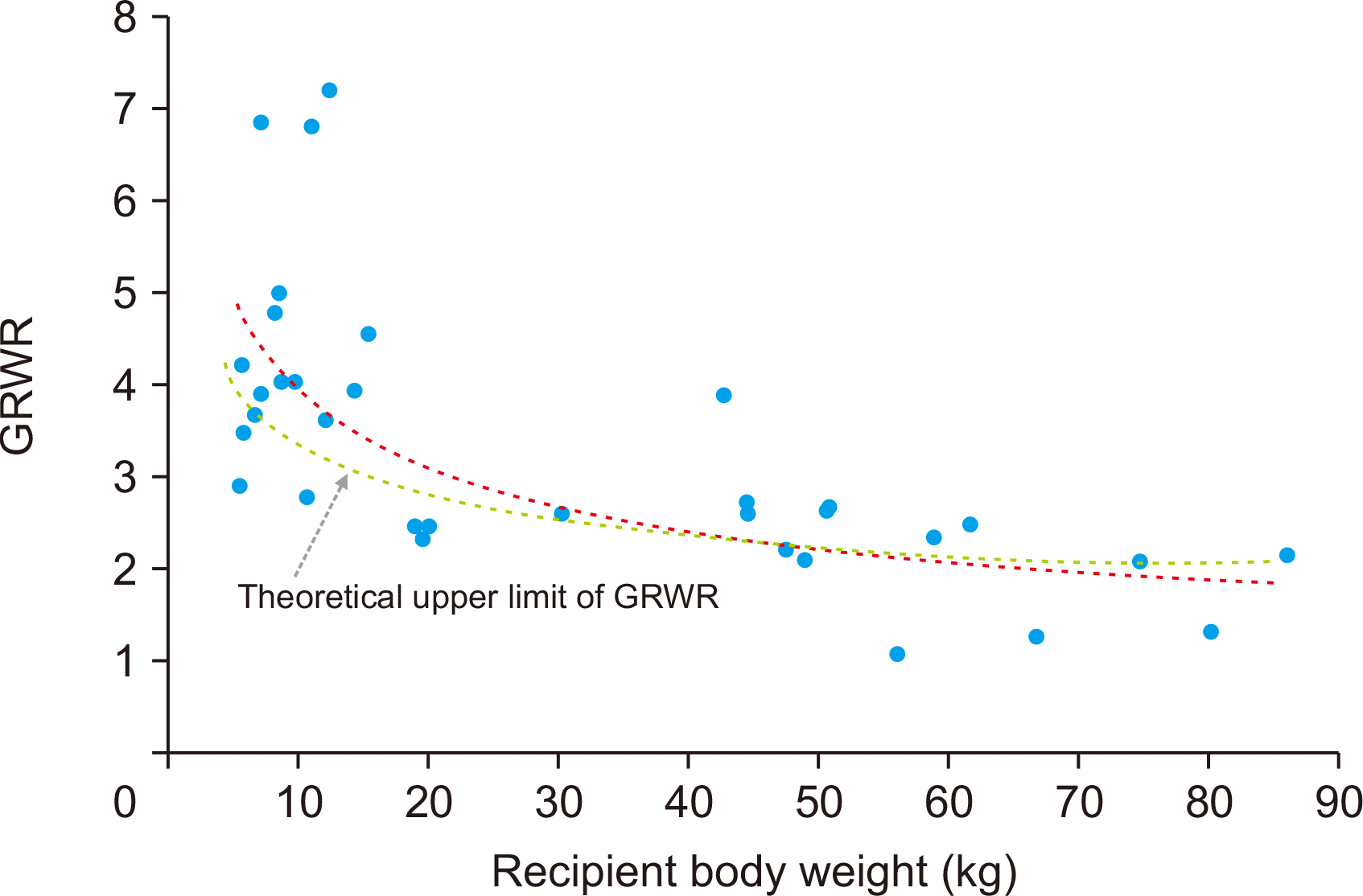
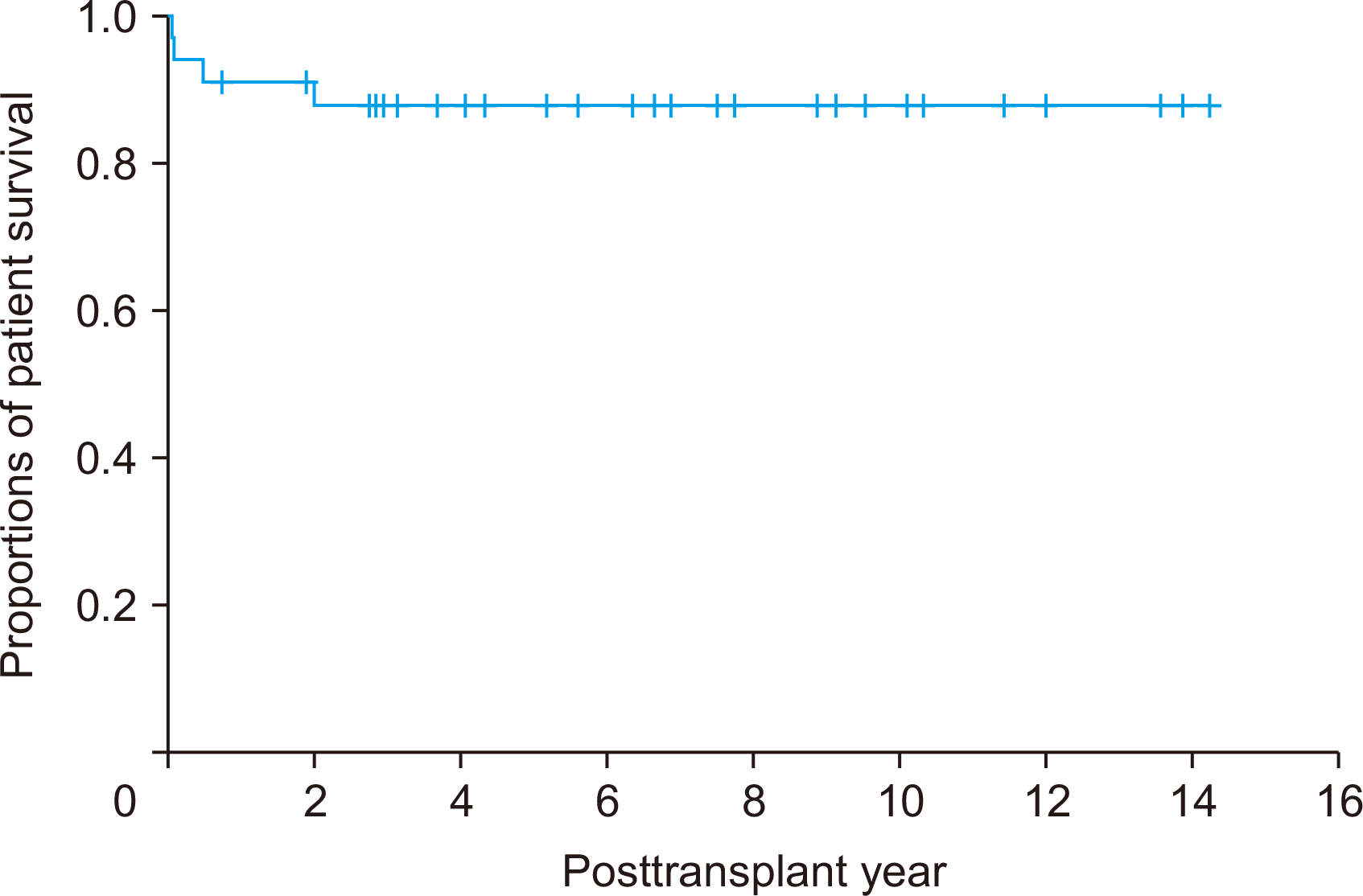
The present study included 22 cases of pediatric-to-pediatric and 12 cases of adult-to-pediatric DDLT using whole liver grafts. During the 20-year study period, there were 348 cases of pediatric LTs including living donor LT in 250 and DDLT in 98 with inclusion of split LT in 64 and whole liver graft LT in 34. Thus, the proportion of whole liver graft LT was 9.8% in pediatric LT cases. There were also a total of 1,056 cases of DDLT, thus pediatric whole liver graft LT occupied 3.2% of all DDLTs. These proportions indicate that whole liver graft LT is the least common form of pediatric LT. The body weight of the deceased donors and pediatric recipients were well matched, thus high correlation between the recipient’s body weight and allograft’s weight and reasonable GRWR were observed.
We previously demonstrated that, of the 31 pediatric donor liver allografts, nine whole liver grafts were implanted in pediatric recipients, 16 whole liver grafts in adult recipients, and 10 split liver grafts in four pediatric and six adult patients [
6]. In our previous study, more than half of the pediatric donor liver grafts were allocated to adult patients. Considering that adult-to-pediatric DDLT occupied one-third of pediatric whole LT and more than half of the pediatric donor liver grafts were used as pediatric-to-adult DDLT, pediatric liver grafts from older children were more frequently used for adult patients than pediatric patients, probably due to donor-recipient body weight matching and lack of pediatric patients with high-priority on the pediatric waiting list. Our previous study and the present study demonstrate the real-world status of DDLT for pediatric donors and recipients in Korea.
According to the Korean standardized growth patterns of children, the 50th percentiles of body weight at 5 years, 10 years, and 15 years are 19.0 kg, 35.5 kg, and 60.1 kg, respectively. Thus, pediatric donors over 10 years of age have comparable body weight to that of adult patients. According to the KONOS regulations of donor-recipient body weight match ratio of 1: 2–1: 2, the liver graft from adolescent donors can be allocated to adult recipients as whole or split liver grafts. To the best of our knowledge, there is still no KONOS regulation for the priority allocation of pediatric liver allografts to pediatric recipients.
In the present study, for pediatric patients aged ≤6 years of age, a high correlation was observed between donor and recipient age, which resulted in the reasonable matching of donor and recipient body weight and GRWR values. On the contrary, pediatric patients aged more than 10 years received liver grafts more frequently from adult donors than pediatric donors, primarily because the incidence of deceased donors of that age is lowest, as shown in our previous analysis of the KONOS database [
6].
Graft-recipient size matching to avoid large-for-size graft implantation is one of the main concerns for young pediatric recipients and avoidance of small-for-size graft implantation is important for adolescent and adult recipients [
10,
11]. The results of this study revealed that the KONOS regulations on donor-recipient body weight ratio appeared to be too wide for accurate size matching, but it was reasonably acceptable for real-world liver organ allocation because severe size mismatching was selectively precluded by the transplant teams.
The incidence of vascular complications reported in the pediatric LT literature is variable and can be up to 25%–33% [
12-
14]. Hepatic artery thrombosis is the most serious complication after LT, and early hepatic artery thrombosis is the main cause of graft loss in pediatric LT. A similar incidence of early vascular complication in the pediatric-to-pediatric LT group and the pediatric-to-adult LT group has been reported, and the low body weight of the recipient was identified as an independent risk factor for vascular complications in pediatric LT [
4]. A meta-analysis compared the survival rates and incidence of surgical complications between pediatric whole LT and other types of LT, in which the graft and patient survival rates were higher in the whole LT group; the incidence of portal vein thrombosis and biliary complications were lower in the whole LT group and the incidence of hepatic artery thrombosis was comparable between the two groups [
1].
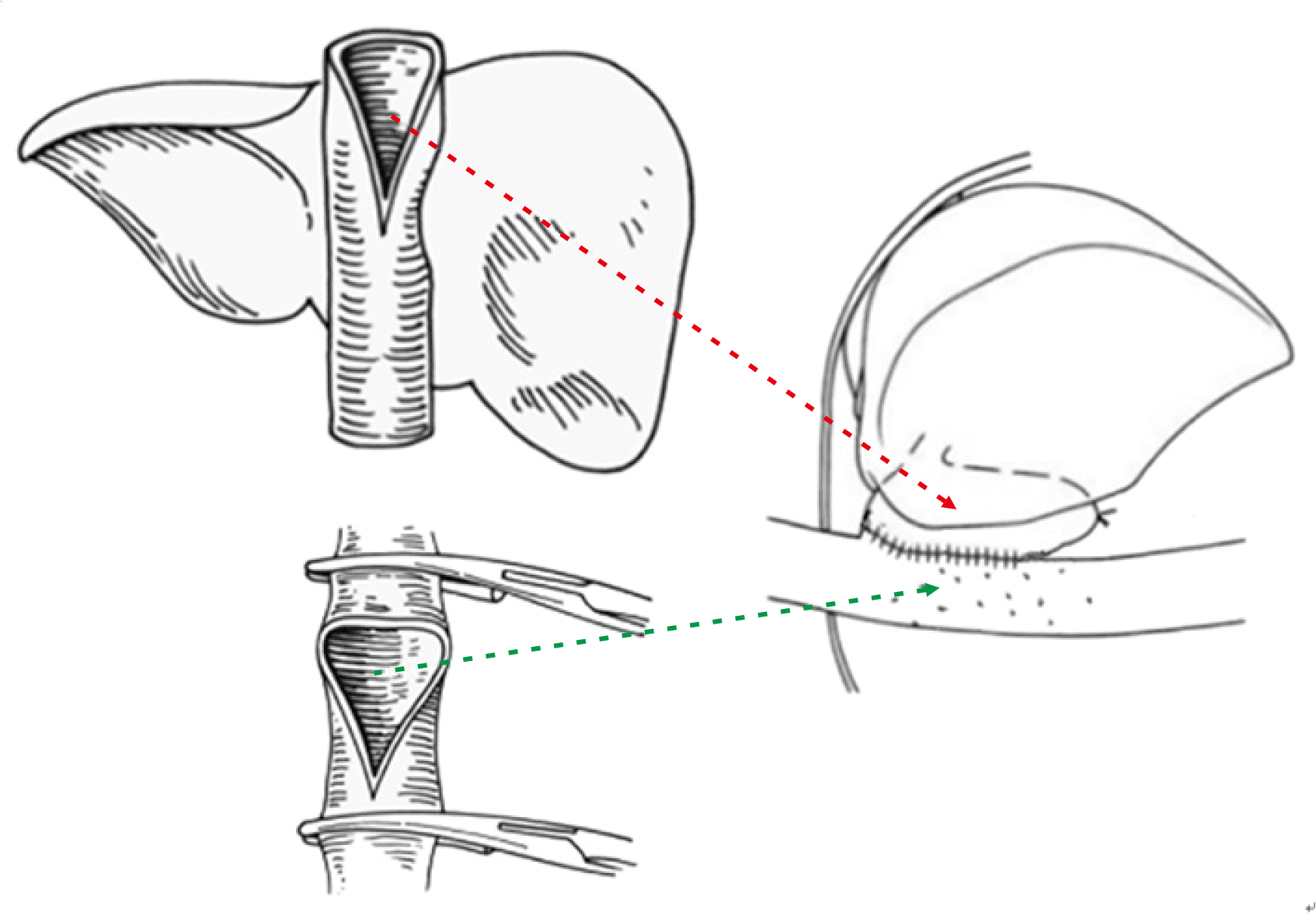
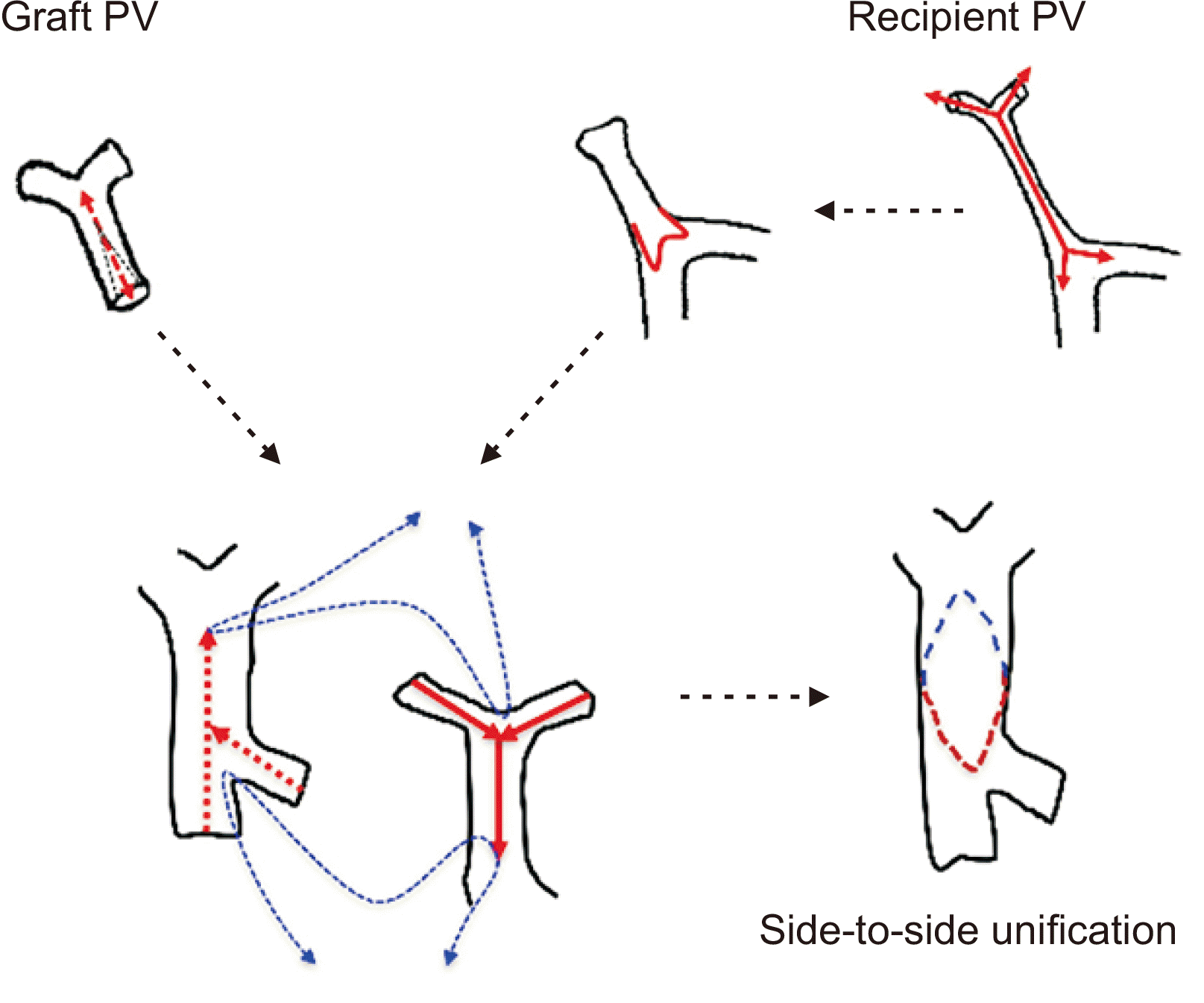
Vascular complications frequently occur following the implantation of a whole liver graft in an infant recipient because of the small vessel size per se although the graft size is well-matched to the recipient body size. We previously demonstrated the occurrence of portal vein complications in four of seven cases with infant-to-infant whole LT, and successful prevention through customized surgical techniques with side-to-side unification venoplasty [
9].
The pediatric end-stage liver disease (PELD) scores used in pediatric patients are usually lower than the model for end-stage liver disease (MELD) scores used in adult patients. Considering the characteristics of liver diseases in childhood, pediatric patients with PELD scores cannot directly compete with adult patients with MELD scores. Many grave conditions that require LT in children do not show abnormal liver function [
2]. In this study with a study period of 20 years, 12 cases of pediatric whole LT were performed in the form of adult-to-pediatric DDLT, but there were only three cases of adult-to-pediatric whole LT after the adoption of MELD score for organ allocation in 2016. The results of this study and our previous study on pediatric deceased donors reveal that pediatric patients are more disadvantageous concerning organ allocation under the current MELD/PELD score-based KONOS allocation system compared to the previous situation [
6].
The Organ Procurement and Transplantation Network (OPTN) of North America prioritizes pediatric potential transplant recipients while allocating livers from pediatric deceased donors [
15]. The ethical principles behind their pediatric organ allocation policy are elucidated by the OPTN/United Network for Organ Sharing Pediatric Transplantation and Ethics Committees [
16], which states that the National Organ Transplant Act charges the OPTN to recognize the differences in health and organ transplantation issues between children and adults throughout the system and adopt criteria, policies, and procedures that address the unique health care needs of children.
We contemplate that it is reasonable to add a PELD score exception for patients with inborn errors of metabolism, hepatoblastoma, and some unusual diseases to the current MELD/PELD score-based allocation system. Considering the relatively small number of such patients at the pediatric DDLT waiting list, we hypothesize that a policy of “pediatric donor liver grafts to pediatric recipients with priority” will be effective to shorten the pediatric waiting list [
2].
This study had a notable limitation. This was a retrospective, single-center study with a relatively small number of study patients. Further high-volume multicenter studies are necessary to validate the results of this study.
In conclusion, the results of this study revealed that KONOS regulations with the matching of donor-recipient body weight worked well and older pediatric patients also received whole liver grafts from adult donors. Considering the adult-favoring reciprocal trades of liver organs among pediatric and adult donors and recipients, it is necessary to establish a policy for pediatric donor liver grafts to pediatric recipients on a priority basis.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download