Abstract
Backgrounds/Aims
Patients with Ampulla of Vater cancer have a better prognosis than those with other periampullary cancers. This study aimed to determine the prognostic impact of lymph node metastasis on survival in patients with ampulla of Vater cancer after surgical resection.
Methods
From 1991 to 2016, we retrospectively reviewed data on 104 patients with ampulla of Vater cancer who had received pancreaticoduodenectomy. Clinicopathologic factors such as lymph node ratio (LNR) and number of metastatic lymph nodes that influence survival were statistically analyzed.
Results
5-year survival rate after resection was 57.8%. Mean number of retrieved and metastatic lymph nodes was 13 and 0.95, respectively. In patients with lymph node metastasis, the median number of metastatic lymph nodes and was 1, and the mean LNR was 0.18. LNR >0.2 was a significant prognostic factor for overall survival. Patients with 0 or 1 metastatic lymph nodes had better survival than those with ≥2 metastatic lymph nodes. Univariate analysis revealed that histologic differentiation of tumor, lymph node metastasis, and T stage were significant prognostic factors for overall survival. Multivariate analysis revealed that tumor differentiation and number of metastatic lymph nodes were independent prognostic factors for survival.
Conclusions
Pancreaticoduodenectomy is an appropriate surgical procedure with acceptable long-term survival for ampulla of Vater cancer. Patients with LNR >0.2 and ≥2 positive lymph node metastasis had a poor survival. Tumor differentiation and ≥2 metastatic lymph nodes were independent significant prognostic factors for overall survival. Curative resection with lymph node dissection might control lymph node spread and enhance survival outcomes.
Ampulla of Vater cancer is an uncommon malignancy among gastrointestinal neoplasms.1 It is defined as a malignant neoplasm involving the papilla of Vater, formed by the union of the duodenum, the pancreatic duct, and the common bile duct. Patients with Ampulla of Vater cancer have a better prognosis than those with other periampullary malignancies after surgical resection.2,3 Pancreatoduodenectomy is the preferred procedure for curative resection. However, the role of adjuvant chemotherapy has not been elucidated.
Various prognostic factors influence survival after surgical resection for Ampulla of Vater cancer. Lymph node metastasis is a well-known significant prognostic factor in periampullary malignancy.4,5 In the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, N stage was divided into N0, N1, and N2. N1 is defined as the presence of 1-3 metastatic lymph nodes, while N2 is defined as the presence of >3 metastatic lymph nodes.6 Several lymph node parameters, such as number of metastatic lymph nodes, total number of retrieved lymph nodes, and lymph node ratio (LNR), have been studied to predict overall survival.5,7
This study aimed to determine the prognostic impact of lymph node metastasis on survival in patients with ampulla of Vater cancer after surgical resection. The impact of metastatic LNR and number of lymph node metastasis on survival was investigated.
This study enrolled 128 patients with ampulla of Vater cancer who underwent surgery. We retrospectively evaulated demographic, clinical, and pathologic data from Korea University Medical Center Guro Hospital from 1991 to 2016. Patients who were diagnosed with ampulla of Vater cancer by pathological examination and underwent R0 resection for curative and margin free resection as well as Whipple’s operation or pylorus-preserving pancreaticoduodenectomy were included.
Patients with underwent R1 or R2 resection and transduodenal ampullectomy were excluded. Compared to pancreaticoduodenectomy, transduodenal ampullectomy is more limited in achieving appropriate lymph node dissection. Finally, 104 patients were included in this study.
The characteristics of all pathologic specimens were evaluated by pathologists at Korea University Medical Center Guro Hospital. The following clinicopathological factors were reviewed: age, sex, tumor size, tumor ulceration, tumor infiltration, tumor differentiation, vascular and perineural invasion, lymph node metastasis, number of metastatic lymph nodes, number of retrieved lymph nodes, LNR, T stage, TMN stage, and administration of adjuvant therapy.
We especially focused on the lymph node status, which includes lymph node metastasis, number of metastatic lymph nodes, and LNR. Lymph node metastasis is categorized based on the presence or absence of metastasis and number of metastatic lymph nodes. LNR is defined as the ratio of metastatic lymph nodes to total resected lymph nodes. We analysed the patients who received pancreaticoduodenectomy. And the lymph nodes were retrieved were peripancreatic, paraduodenal lymph nodes, retropancreatic lymph nodes and lymph nodes along the hepatic arterh, portal vein, hepatoduodenal ligament.
Pathological findings and TNM classification were staged according to the 8th edition of the AJCC for ampullary carcinoma.6
All statistical analyses were carried out using IBM SPSS v 25.0 (SPSS, Inc, Chicago, IL). Clinicopathologic factors such as LNR and number of metastatic lymph nodes that influence survival were statistically analyzed using the Kaplan–Meier method. The chi-square test was used to compare the study groups. Overall survival and disease-free survival rates were estimated using the Kaplan–Meier method. The Cox proportional hazard model was used to identify factors that influence survival. Statistical significance was set at p<0.05.
This study included 104 patients who underwent pancreaticoduodenectomy and R0 operation for Ampulla of Vater cancer. Patients who received underwent R1 resection, R2 resection, or transduodenal ampullectomy were excluded.
Well-differentiated tumors were found in 38 patients, and moderately or poorly differentiated tumors were found in 66 patients. Perineural invasion was found in 15 patients. Patients were categorized into stage I (n=36), II (n=26), and III (n=42) according to the AJCC TNM staging system. Furthermore, 18, 25, and 61 patients had T1, T2, and T3 stage tumors, respectively. The mean number of retrieved and metastatic lymph nodes was 13 (±8) and 0.95 (±1.9), respectively. In patients with lymph node metastasis (N1 or N2), the median number of metastatic and retrieved lymph nodes was 1 (range, 1-13) and 15 (range, 8-40), respectively. The median LNR in patients with metastatic lymph node was 0.18 (±0.18).
Fig. 1 shows the overall and disease-free survival curve of patients with Ampulla of Vater cancer after surgery. The 3-year and 5-year overall survival rate (YSR) was 72% and 57.8%, respectively. The 3-year and 5-year disease-free survival rate was 60.3% and 49.9%, respectively. Recurrence was seen in 48 patients. As shown in Table 1, lymph node status, lymph node metastasis, number of metastatic lymph node, and LNR are strong factors that affect overall survival.
Univariate analysis was performed to identify predictors of overall survival among clinicopathological factors (Table 1). In univariate analysis, tumor differentiation (well/moderate and poor, p=0.010), perineural invasion (p=0.068), lymph node metastasis (p=0.008), number of metastatic lymph nodes (0 or 1/≥2, p=0.004), LNR (≤0.2/ >0.2, p=0.002), T stage (T1/T2/T3, p=0.014), and TNM stage (I/II/III, p=0.001) were identified as important prognostic factors for overall survival.
Factors that had a p-value of <0.1 in univariate analysis were included in multivariate analysis. Tumor differentiation, perineural invasion, and number of metastatic lymph nodes were included in the multivariate analysis (Table 2). Tumor differentiation (hazard ratio [HR], 2.021; confidence interval [CI], 1.013-4.031; p=0.046) and number of metastatic lymph nodes (HR, 2.285; CI, 1.191- 4.382; p=0.013) were identified as independent prognostic factors for overall survival.
Table 1 shows the four factors related to lymph node status; presence of lymph node metastasis, number of metastatic lymph nodes, number of retrieved lymph nodes, and LNR.
In patients with lymph node metastasis, the median LNR was 0.18 (±0.18). Therefore, patients were divided into the LNR >0.2 and LNR ≤0.2 groups based on the median LNR. We selected relatively simple cutoff values to one decimal places for easy application in clinical practice.
The presence of lymph node metastasis was a significant prognostic factor affecting overall survival. The 5YSR in the negative and positive lymph node groups was 64.7% and 48.5%, respectively (p=0.008). The number of retrieved lymph nodes did not affect overall survival. The number of metastatic lymph nodes was a significant prognostic factor affecting overall survival (p=0.004). Patients with 0 or 1 metastatic lymph nodes demonstrated significant better survival than those with the ≥2 metastatic lymph nodes (5YSR, 65.2% vs. 33.9%, p=0.004; Fig. 2A). The 5YSR in the LNR ≤0.2 and LNR >0.2 groups was 60.2% and 26.3% respectively (p=0.002; Fig. 2B). The 5YSR was significantly different among patients with metastatic lymph nodes in the LNR ≤0.2 and LNR >0.2 groups (p=0.013; Fig. 2C).
The prognosis of Ampulla of Vater cancers after R0 resection is better than that of other periampullary malignancy.8 In previous studies, the 5YSR in patients with pancreatic cancer and distal bile duct cancer after surgical resection was 16.1%-24%,7,9,10 and 38.3%-40.8%,4,11,12 respectively. The 5YSR in patients with Ampulla of Vater cancer after surgery in our study was 57.8%. In previous studies, the 5YSR in patients with Ampulla of Vater was 51.1%-61%,8,13,14 similar to our result. Thus, pancreaticoduodenectomy is a standard surgical procedure with acceptable long-term survival for patients with ampulla of Vater cancer. Complete lymph node dissection is an important part of R0 resection.
In this study, univariate analysis revealed that presence of lymph node metastasis, number of metastatic lymph nodes, LNR, and stage were significant prognostic factors for overall survival. In multivariate analysis, tumor differentiation and number of metastatic lymph nodes were independent prognostic factors for overall survival.
Lymph node metastasis was found to be a strong predictor of survival and recurrence.5,8,11,13,15 In other studies, tumor differentiation was also found to be a significant prognostic factor for survival.8,14,16 In one study, moderate-to-poor tumor differentiation was an independent predictive factor for lymph node metastasis.15 Zhou et al.16 have reported a strong relationship between tumor differentiation and lymph node metastasis in patients with ampulla of Vater cancer. Lymph node metastasis is associated with aggressive tumor characteristics. In another study, there was a significant relationship between lymph node metastasis and gross appearance of the tumor (protruding, mixed, ulcerative). Mixed and ulcerative types of tumors have a greater proportion of metastatic lymph nodes than protruding type of tumors. A significant relationship between tumor invasion depth and lymph node involvement has been identified.17
The number of metastatic lymph nodes also affect overall survival. Kang et al. studied the lymph node metastasis assessed in the Surveillance, Epidemiology and End Results database in 1057 ampullary adenocarcinomas. They reported that two significant cutoff points for metastatic lymph nodes (0 and 2) were used to categorize the patients into three groups with clinically important differences in median survival—patients without lymph node metastasis (median survival, 91 month), patients with 1-2 metastatic lymph nodes (median survival, 29 months), and patients with ≥3 metastatic lymph nodes (median survival, 19 months).5 Balci et al. studied 313 patients who underwent pancreatoduodenectomy for ampullary adenocarcinoma categorized as N0, N1 (1-2 metastatic lymph nodes), or N2 (≥3 metastatic lymph nodes). They reported significantly different survival rates among these patients.18 In other studies, although using different categories for the number of metastatic lymph nodes, it was significantly predictive for overall survival.16,19 In our study, patients with 0-1 metastatic lymph nodes had significantly different survival rates than those with ≥2 metastatic lymph nodes. In our study, patients with 0 and 1 metastatic lymph node had similar survival rates. Thus, the prognosis in patients with 2 or 3 metastatic lymph nodes was different from the prognosis in patients with one metastatic lymph node.
According to 8th edition of the AJCC, regional lymph nodes of ampulla of Vater cancer are peripancreatic lymph nodes, including the lymph nodes along the hepatic artery and portal vein.6 A minimum of 12 lymph nodes should be included in the pancreaticoduodenectomy specimen for optimal histologic examination. LNR is defined as the ratio of the number of positive lymph nodes to the number of retrieved lymph nodes. LNR may reflect the real status of lymph nodes. LNR can stratify the prognosis in subgroups of patients with similar metastatic lymph nodes.7 LNR is also an important prognostic factor for pancreatic, stomach, and colon cancers.7,9,20,21 Several studies have reported the prognostic significance of LNR.8,13,22 In a study by Hsu et al., LNR >0.056 predicted a poor disease-free and overall survival rate after radical surgery (HR, 4.3; p<0.001); the median overall survival in patients with LNR ≤0.056 and LNR >0.056 was 84.9 months and that in patients with ≤20 retrieved lymph nodes was 25.5 months (p<0.001).13
Kwon et al. studied lymph node parameters affecting survival in ampulla of Vater cancer, such as number of metastatic lymph nodes, total number of lymph nodes, and LNR. In a previous study, categorizing the LNR ≤17% vs. >17%, multivariate analysis revealed that among the lymph node parameters, LNR was able to independently predict survival (HR, 2.12; p<0.022) and recurrence (HR, 3.38; p=0.004).8 In other study, LNR ≥0.015 was associated with distant recurrence after surgical resection of ampulla of Vater cancer. Thus, adjuvant therapy is warranted in patients with LNR ≥0.015.22
In a study by Sakata et al., univariate analysis revealed that number of metastatic lymph nodes (0, 1-3, or ≥3; p<0.001) and LNR (0, 0-0.1, or >0.1; p<0.001) were significant prognostic factors for survival. However, in multivariate analysis, only number of metastatic lymph nodes was an independent prognostic factor (p<0.001).19 Similarly in the study by Zhou et al.,16 LNR (0, 0-0.2, >0.2) was a useful factor for predicting the prognosis of radical surgery for ampulla of Vater cancer, although number of metastatic lymph nodes (0, 1, 2, 3, and ≥4) was the most important factor.
In the literature, most studies have suggested the prognostic impact of number of metastatic lymph nodes and LNR, but the cutoff value differed between the studies. In our study, patients with LNR >0.2 had significantly poorer survival in the univariate analysis, but not in the multivariate analysis. Further, ≥2 metastatic lymph nodes was an independent prognostic factor for overall survival. This simple cutoff value is practical to use in clinical settings. In our study, patients with 0 and 1 metastatic lymph node had similar survival rates. The less aggressive biology of ampulla of Vater cancer makes LNR more important for sub-stratification, especially considering that most patients with N1 disease also have 1 or 2 metastatic lymph nodes.8 Several limitations of this study exists. It was retrospective, including long period with small number of patients. There is a possibility that the presence or absence of additional treatment after radical surgery has affected the survival. However, the adjuvant therapy after pancreaticoduodenectomy was not strictly protocol driven and administered at the discretion of the surgeons and oncologists in this study. Therefore clearcut analysis was not possible focused on the efficacy of the adjuvant therapy according to the lymph node metastatsis, LNR.
In conclusion, patients with ampulla of Vater cancer after curative resection demonstrated acceptable survival rates (5YSR, approximately 60%). Patients with LNR >0.2 and metastatic lymph nodes ≥2 had poor survival. In multivariate analysis, number of metastatic lymph nodes and tumor differentiation were independent prognostic factors for overall survival. Curative resection with complete lymph node dissection might control lymph node spread and enhance survival outcomes. Greater awareness of the impact of number of metastatic lymph nodes and LNR may help clinicians provide appropriate adjuvant treatment. Further, it will help in the close monitoring of patients at a high risk of recurrence.
REFERENCES
1. Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. 1998; Factors predictive of survival in ampullary carcinoma. Ann Surg. 228:87–94. DOI: 10.1097/00000658-199807000-00013. PMID: 9671071. PMCID: PMC1191432.



2. Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. 2008; Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 207:210–218. DOI: 10.1016/j.jamcollsurg.2008.01.028. PMID: 18656049.


3. Lazaryan A, Kalmadi S, Almhanna K, Pelley R, Kim R. 2011; Predictors of clinical outcomes of resected ampullary adenocarcinoma: a single-institution experience. Eur J Surg Oncol. 37:791–797. DOI: 10.1016/j.ejso.2011.06.008. PMID: 21741199.


4. Kiriyama M, Ebata T, Aoba T, Kaneoka Y, Arai T, Shimizu Y, et al. 2015; Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 102:399–406. DOI: 10.1002/bjs.9752. PMID: 25611179.

5. Kang HJ, Eo SH, Kim SC, Park KM, Lee YJ, Lee SK, et al. 2014; Increased number of metastatic lymph nodes in adenocarcinoma of the ampulla of Vater as a prognostic factor: a proposal of new nodal classification. Surgery. 155:74–84. DOI: 10.1016/j.surg.2013.08.004. PMID: 24238122.


6. Amin MB, Edge S, Greene F, Byrd DR Byrd, Brookland RK, Washington MK, et al. 2017. AJCC cancer staging manual. 8th ed. Springer;New York: DOI: 10.1007/978-3-319-40618-3.
7. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. 2007; Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 141:610–618. DOI: 10.1016/j.surg.2006.12.013. PMID: 17462460.


8. Kwon J, Kim K, Chie EK, Kim BH, Jang JY, Kim SW, et al. 2017; Prognostic relevance of lymph node status for patients with ampullary adenocarcinoma after radical resection followed by adjuvant treatment. Eur J Surg Oncol. 43:1690–1696. DOI: 10.1016/j.ejso.2017.05.024. PMID: 28648977.


9. Malleo G, Maggino L, Capelli P, Gulino F, Segattini S, Scarpa A, et al. 2015; Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the standard International Study Group of Pancreatic Surgery definition of lymphadenectomy for cancer. J Am Coll Surg. 221:367–379. e4. DOI: 10.1016/j.jamcollsurg.2015.02.019. PMID: 26081176.


10. Robinson SM, Rahman A, Haugk B, French JJ, Manas DM, Jaques BC, et al. 2012; Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 38:333–339. DOI: 10.1016/j.ejso.2011.12.020. PMID: 22317758.


11. Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, et al. 2014; Prognostic factors for survival after curative resection of distal cholangiocarcinoma: perineural invasion and lymphovascular invasion. Surg Today. 44:1879–1886. DOI: 10.1007/s00595-014-0846-z. PMID: 24535697.
12. Choi SB, Park SW, Kim KS, Choi JS, Lee WJ. 2009; The survival outcome and prognostic factors for middle and distal bile duct cancer following surgical resection. J Surg Oncol. 99:335–342. DOI: 10.1002/jso.21238. PMID: 19226533.


13. Hsu CH, Chen TD, Tsai CY, Hsu JT, Yeh CN, Jan YY, et al. 2015; Prognostic value of the metastatic lymph node ratio in patients with resectable carcinoma of ampulla of Vater. Medicine (Baltimore). 94:e1859. DOI: 10.1097/MD.0000000000001859. PMID: 26496337. PMCID: PMC4620839.

14. Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. 2012; Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 105:266–272. DOI: 10.1002/jso.22090. PMID: 21882202.


15. Okano K, Asano E, Kushida Y, Kamada H, Mori H, Suzuki Y. 2014; Factors influencing lymph node metastasis in patients with ampullary adenocarcinoma. Dig Surg. 31:459–467. DOI: 10.1159/000370251. PMID: 25613423.


16. Zhou J, Zhang Q, Li P, Shan Y, Zhao D, Cai J. 2013; Prognostic relevance of number and ratio of metastatic lymph nodes in resected carcinoma of the ampulla of Vater. Chin J Cancer Res. 25:735–742. DOI: 10.3978/j.issn.1000-9604.2013.12.03. PMID: 24385702. PMCID: PMC3872556.


17. Kayahara M, Ohta T. 2010; Gross appearance of the ampullary tumor predicts lymph node metastasis and outcome. Dig Surg. 27:127–131. DOI: 10.1159/000286839. PMID: 20551657.


18. Balci S, Basturk O, Saka B, Bagci P, Postlewait LM, Tajiri T, et al. 2015; Substaging nodal status in ampullary carcinomas has significant prognostic value: proposed revised staging based on an analysis of 313 well-characterized cases. Ann Surg Oncol. 22:4392–4401. DOI: 10.1245/s10434-015-4499-y. PMID: 25783680. PMCID: PMC4575255.



19. Sakata J, Shirai Y, Wakai T, Ajioka Y, Akazawa K, Hatakeyama K. 2011; Assessment of the nodal status in ampullary carcinoma: the number of positive lymph nodes versus the lymph node ratio. World J Surg. 35:2118–2124. DOI: 10.1007/s00268-011-1175-7. PMID: 21717240.


20. Jiang C, Wang F, Guo G, Dong J, Liu S, He W, et al. 2019; Metastatic lymph node ratio as a prognostic indicator in patients with stage IV colon cancer undergoing resection. J Cancer. 10:2534–2540. DOI: 10.7150/jca.29216. PMID: 31258759. PMCID: PMC6584347.



21. Kutlu OC, Watchell M, Dissanaike S. 2015; Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg Oncol. 24:84–88. DOI: 10.1016/j.suronc.2015.03.001. PMID: 25912951.


22. Roland CL, Katz MH, Gonzalez GM, Pisters PW, Vauthey JN, Wolff RA, et al. 2012; A high positive lymph node ratio is associated with distant recurrence after surgical resection of ampullary carcinoma. J Gastrointest Surg. 16:2056–2063. DOI: 10.1007/s11605-012-2015-2. PMID: 22914983. PMCID: PMC5131719.



FIGURES AND TABLES
Fig. 1
Overall survival (A) and disease survival curve (B) of patients with Ampulla of Vater cancer after surgical resection.
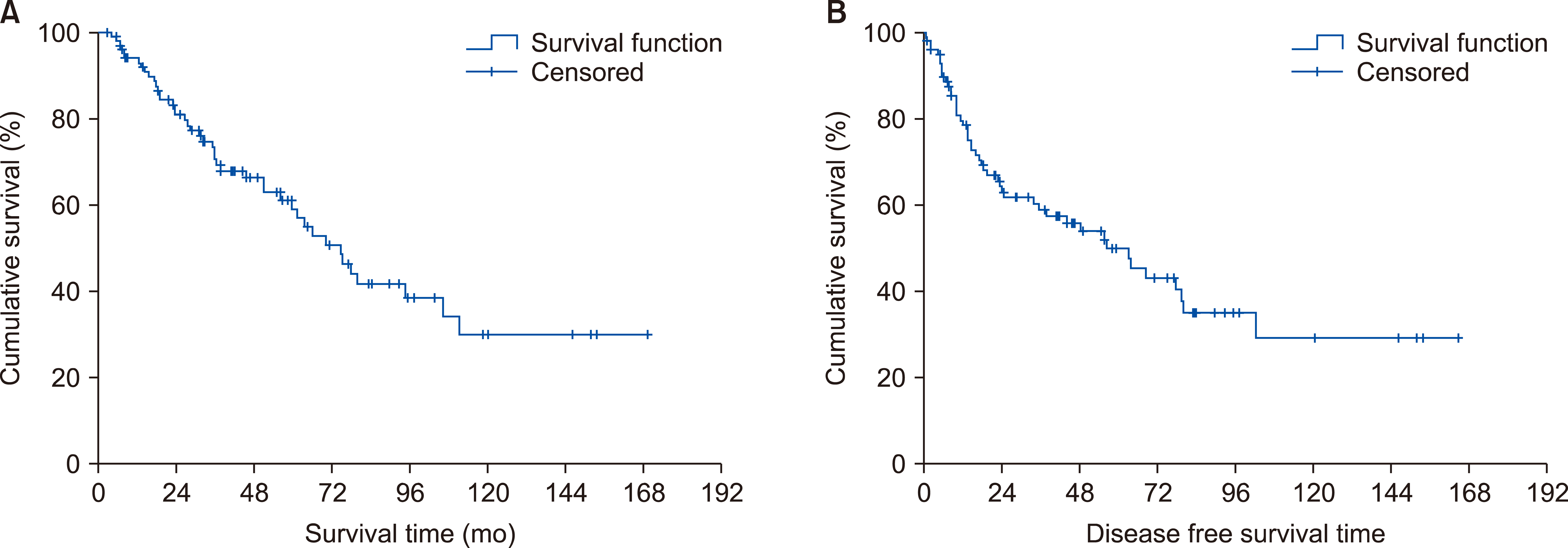
Fig. 2
Overall survival curve according to the number of metastatic lymph nodes (A). Overall survival curve according to lymph node ratio (B). Overall survival curve according to lymph node ratio in the lymph nodes positive patients (C).
Table 1
Univariate analysis of clinicopathological factors affecting overall survival in patients with ampulla of Vater cancer following surgical resection
Table 2
Multivariate analysis of clinicopathological factors affecting overall survival in patients with ampulla of Vater cancer following surgical resection




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download