Abstract
Backgrounds/Aims
Despite advances in surgical techniques and perioperative supportive care, radical resection of hilar cholangiocarcinoma is the only modality that can achieve long-term survival. We chronologically investigated surgical and oncological outcomes of hilar cholangiocarcinoma and analyzed the factors affecting overall survival.
Methods
We retrospectively enrolled 165 patients with hilar cholangiocarcinoma who underwent liver resection with a curative intent. The patients were divided into groups based on the period when the surgery was performed: period I (2005-2011) and period II (2012-2018). The clinicopathological characteristics, perioperative outcomes, and survival outcomes were analyzed.
Results
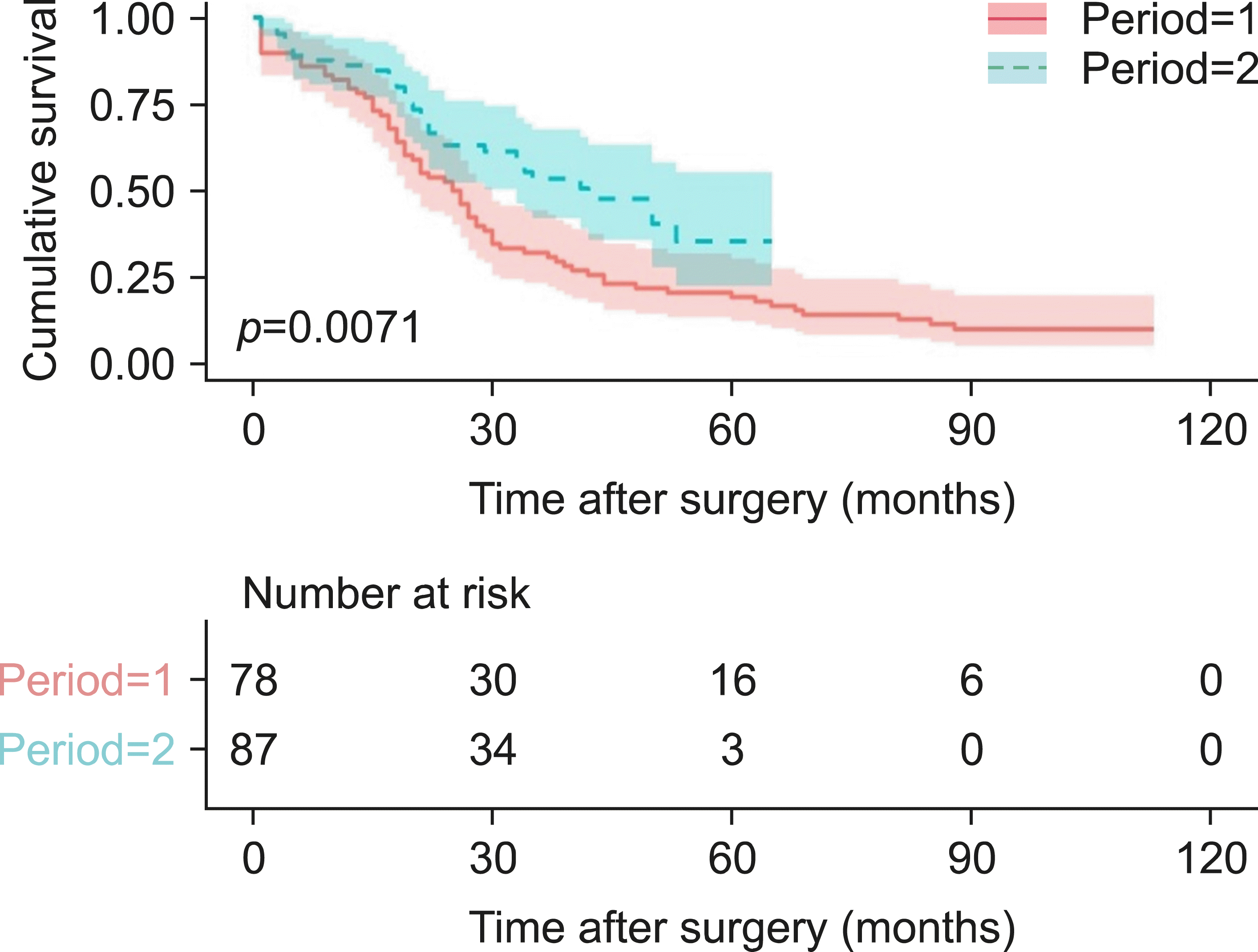
The patients’ age, serum CA19-9 levels, and serum bilirubin levels at diagnosis were significantly higher in the period I group. There were no differences in pathological characteristics such as tumor stage, histopathologic status, and resection status. However, perioperative outcomes, such as estimated blood loss (1528.8 vs. 1034.1 mL, p=0.020) and postoperative severe complication rate (51.3% vs. 26.4%, p=0.022), were significantly lower in the period II group. Regression analysis demonstrated that period I (hazard ratio [HR]=1.591; 95% confidence interval [CI]=1.049-2.414; p=0.029), preoperative serum bilirubin at diagnosis (HR=1.585; 95% CI=1.058-2.374; p=0.026), and tumor stage (III, IV) (HR=1.671; 95% CI: 1.133-2.464; p=0.010) were significantly associated with poor prognosis. The 5-year survival rate was better in the period II patients than in the period I patients (35.1% vs. 21.0%, p=0.0071).
Surgical resection is the only treatment modality that can achieve long-term survival outcomes after the treatment of hilar cholangiocarcinoma, a malignancy of the biliary epithelium of the hilum.1,2 Despite advances in surgical techniques and perioperative supportive care, the treatment of hilar cholangiocarcinoma remains challenging. Due to its infiltrative nature by longitudinal extension and its proximity to vital vascular structures, surgical resection of the tumor is limited and has unfavorable oncological outcomes.3,4 Extended major hepatectomy with concomitant vascular and biliary resection and reconstruction is associated with high perioperative morbidity and mortality rates, and, as such, the evolution of surgical treatment for hilar cholangiocarcinoma is ongoing.
To date, the actual 5-year survival rate for hilar cholangiocarcinoma is 14%-45%.5,6 In addition, the prognostic factors affecting long-term survival include lymph node metastasis, tumor resection margin status, and histological differentiation.7,8 Multicenter studies have reported that the broad spectrum of oncological outcomes originates from the variation in follow-up periods, inclusion of palliative resection, and numerous surgical approaches. Furthermore, there have been limited reports on the chronological analysis of hilar cholangiocarcinoma.9,10
Therefore, in the current study, we aimed to chronologically investigate the surgical and oncological outcomes of hilar cholangiocarcinoma using a single-center cohort and analyzed the factors affecting overall survival (OS).
We retrospectively reviewed the medical records of 165 patients who underwent surgical treatment with a curative intent for hilar cholangiocarcinoma from January 2005 to March 2018 at our hospital. The patients were divided into two groups according to the period when they underwent surgery: period I (2005-2011; n=78) and period II (2012- 2018; n=87). The surgical treatment comprised liver resection, including more than three segments, with caudate lobectomy and Roux-en-Y hepaticojejunostomy. The types of surgery were right hemihepatectomy and extended right hemihepatectomy, left hemihepatectomy and extended left hemihepatectomy, and central bisectionectomy.
The need for informed consent was waived due to the retrospective nature of the study and because anonymous clinical data were used for the analyses. The study was approved by the institutional review board of the hospital (approval number: 4-2020-0676).
The type of hilar cholangiocarcinoma was classified according to the Bismuth-Corlette classification.11 The final pathology and stage were analyzed according to the American Joint Committee on Cancer, 8th edition. R0 resection indicates a microscopically margin-negative resection, in which there was no gross or microscopic tumor remaining at the primary tumor bed as described in the final pathologic report. R1 resection indicates the removal of all macroscopic diseases, but with microscopic margins positive for tumors. Postoperative complications were classified according to the Clavien-Dindo classification.12 Postoperative liver failure was described in accordance with the classification criteria of the International Study Group of Liver Surgery.13
All patients underwent computed tomography (CT) and magnetic resonance imaging preoperatively. Portal vein embolization for remnant liver volume expansion was performed according to the remnant liver volume on CT scan- based liver volumetry before the planned surgery. The remnant liver volume was re-evaluated using CT scans obtained 2 weeks after porta vein embolization. If patients demonstrated severe obstructive jaundice with cholangitis, we considered preoperative biliary drainage, including percutaneous transhepatic biliary drainage, endoscopic retrograde biliary drainage, and endoscopic nasobiliary drainage, depending on the situation. The standard serum bilirubin level for performing radical resection is 3-4 mg/dl before surgery; however, this is based on the condition of the liver.
Surgical resection was performed by four surgeons in our institution. Major liver resections, including right hemihepatectomy, extended right hemihepatectomy, left hemihepatectomy, extended left hemihepatectomy, and central bisectionectomy, were performed using caudate lobectomy. For radical resection of the tumor, tumors that were visible by eye were removed, and examination of the proximal and distal margins was performed using frozen sections. For lymph node resection, the hepatic and duodenal ligament lymph nodes, including the right side of the celiac artery and the posterior pancreatic lymph nodes, were subjected to en bloc resection. The remaining lobe after resection was defined as the one with less bile duct invasion and no hepatic artery invasion and leaving the lobe through which healthy bile is drained. A total of 19 patients underwent a concomitant major vascular resection, including hepatic artery/portal vein resection and reconstruction.
Postoperative management of the patients was generally performed in the general ward. Pain was managed using patient-controlled epidural analgesia, and enteral feeding was started on postoperative day 3-5. All patients underwent CT on postoperative day 5. After discharge, routine follow-up imaging was performed at an outpatient clinic and adjuvant therapy was considered depending on the final pathology report.
According to the final pathologic report, patients with R1 resection or higher or T2 grade or higher or those who were positive for retrieved lymph nodes underwent adjuvant chemotherapy. The regimen was selected according to the physician’s preference and/or the patient’s condition. The distribution of the main regimen according to period is summarized in Table 1.
Descriptive analyses of the clinical data were conducted using Student’s t-test and Pearson’s chi-square test. OS was analyzed using the Kaplan-Meier method, and statistical differences were calculated using the log-rank test. OS was calculated from the day of surgery to death or the last follow-up. Statistical significance was set at p< 0.05. Cox proportional hazards regression models were used to determine the association between patient characteristics and OS. All results, except OS analysis, are presented as mean and standard deviation with percentages. The analysis of OS is presented with the median survival (month). To reduce selection bias and the effect of potential confounders, predictive factors were calculated using logistic regression based on age, sex, etiology, surgical procedure, and American Joint Committee on Cancer stage. All statistical analyses were performed using SPSSⓇ for Windows version 22.0 (IBM Corp., Armonk, NY, USA).
In both periods, the patients were predominantly male. The mean patient age was 62.2 and 65.6 years in period I and period II, respectively (p=0.021). There were no statistically significant differences in terms of liver profile laboratory findings, except serum bilirubin level at diagnosis (7.6±6.8 vs. 5.0±5.6 mg/dl, p=0.008). The mean serum CA19-9 level 1 day before surgery was significantly higher in period I than in period II (1306.3 vs. 362.1 U/ml, p=0.039).
Preoperative percutaneous transhepatic biliary drainage and endoscopic biliary drainage were performed in 63 patients (80.8%) and 65 patients (74.7%) in periods I and II, respectively. There were no significant differences in the type of hilar cholangiocarcinoma and the proportion of preoperative portal vein embolization between the two periods. Adjuvant chemotherapy (66.7% vs. 74.7%, p=0.335) and adjuvant radiotherapy (38.5% vs. 36.8%, p=0.951) were performed during period I and period II (Table 2).
During period I, 43 patients (53.8%) underwent right hemihepatectomy, 35 patients (44.9%) underwent left hemihepatectomy, and 8 patients (10.3%) underwent extended left hemihepatectomy. Further, two patients underwent hepatic artery resection and six patients underwent portal vein resection. During period II, 53 patients (59.9%) underwent right hemihepatectomy, of whom, 4 patients (4.6%) underwent extended right hemihepatectomy. None of the patients underwent extended left hemihepatectomy. Central bisectionectomy was performed in two patients (2.3%), hepatic artery resection was performed in five patients (5.7%), and portal vein resection was performed in six patients (6.9%) (Table 3).
The final pathologic report for the entire study period was classified according to American Joint Committee on Cancer, 8th edition. Regarding T stage, T2b was the most common (53.8% vs. 50.6%), followed by T2a (38.5% vs. 31.0%) in period I and period II. During both periods, three patients with no residual tumor in the resected specimen received neoadjuvant chemoradiotherapy. There were no significant differences in the number of positive lymph nodes and retrieved lymph nodes between the two periods. The proportion of node metastasis was 43.6% during period I and 34.4% during period II. Further, 27 (34.6%) and 26 (29.8%) patients had stage III cancer during period I and period II, respectively. R0 resection was performed in 60 patients (79.5%) in period I and 60 patients (69%) in period II. There were no statistically significant differences between the two periods in terms of T stage, N stage, cell differentiation, and histopathologic characteristics (Table 4).
There was no significant difference in hospital stay between the two periods. The estimated blood loss was significantly lower during period II than during period I (1528.8 vs. 1034.1 ml, p=0.020). The proportion of patients who were transfused intraoperatively was also lower during period II than during period I (61.5% vs. 29.9%, p<0.001). The postoperative complications were lower during period II than during period I (p=0.022). In both periods, Clavien- Dindo grade IIIa complication was the most common (33.3% vs. 16.1%). Post-hepatectomy liver failure was not significantly different between the two periods (p=0.594). Regarding postoperative 30-day mortality, six (7.7%) patients in period I and four (4.6%) patients in period II died (Table 5).
The median survival duration was 25 months (95% confidence interval [CI]=19.807-30.193) and 42 months (95% CI=27.540-56.460) in period I and period II, respectively. The 5-year survival rate was 21.0% and 35.1% in period I and period II, respectively. The difference in survival duration was statistically significant (p=0.0071) (Fig. 1).
The univariate and multivariate analyses of factors related to survival are summarized in Table 6. During period I (hazard ratio [HR]=1.591; 95% CI=1.049-2.414; p= 0.029), preoperative serum bilirubin level at diagnosis (HR= 1.585; 95% CI=1.058-2.374; p=0.026), and tumor stage (III and IV) (HR=1.671; 95% CI=1.133-2.464; p=0.010) were significantly associated with poor prognosis. Preoperative serum CA 19-9 level, lymph node metastasis, and adjuvant chemotherapy were significant in univariate analysis but not in multivariate analysis.
Despite the efforts to improve the oncological outcome of hilar cholangiocarcinoma, the actual 5-year survival rate has remained low at 14%-45%.14 In our series, the 5-year survival rate was 35.1% in the period II group and 21.0% in the period I group. Several studies have reported chronological improvement and evolution in the surgical and oncological outcomes of hilar cholangiocarcinoma.15,16 Significant improvements were observed as the period progressed; however, the gap between the two periods is >25 years. Since the investigated period is long, the analysis of contributing prognostic factors can be strongly influenced by various biases. Therefore, it is necessary to analyze the change over a relatively short period and investigate the changes caused by the differences. In this series, we set the analyzing and comparing period as 6 years.
We found that period I was a significant prognostic factor for OS. The significant difference in OS between periods I and II may have resulted from the operative factors and perioperative support. These chronological and oncological improvements in patients with surgically resected hilar cholangiocarcinoma have been shown in previous studies.9,17 Compared to patients in the period I group, those in the period II group had lower serum CA 19-9 levels immediately prior to the surgery. Although there was no statistically significant difference between the two groups, the higher proportion of preoperative cholangitis and neoadjuvant chemotherapy in the period II group may have affected the preoperative CA 19-9 levels. Three patients who received neoadjuvant chemotherapy reported no residual tumor in the final pathologic report. Regarding intraoperative factors, the number of extended right hemihepatectomies and central bisectionectomies in the period II patients was significantly higher than that in the period I patients, whereas extended left hemihepatectomy was performed more frequently in the period I patients. Nevertheless, there were no significant differences in terms of hospital stay, postoperative 30-day mortality, and postoperative liver failure rate; however, the period II group showed better outcomes in terms of estimated blood loss and the blood transfusion and postoperative complication rates. Previous studies have reported that operative morbidity and oncological outcomes of biliary-pancreatic malignancy are associated with intraoperative blood loss and blood transfusion.18-20 The association between blood transfusion and long-term survival after resection for perihilar cholangiocarcinoma has been examined previously.9,21 The specific mechanism leading to the adverse effect of blood transfusion is unclear, but experimental and clinical studies have demonstrated that blood transfusion suppresses host immunity by reducing natural killer cell activity and cytotoxic T cell function.
Regarding radical resection status, the R0 resection rate of the period II group was lower than that of the period I group. Several previous studies that conducted a chronological analysis of the surgical outcomes following the treatment of perihilar cholangiocarcinoma reported that the R0 resection rate was 73%-79%.9,15-17 Since the previous studies included all R2 status, the ratio will be lower in the comparison between R0 and R1 only. However, the rate of R0 resection increased as the period progressed. In the current study, the spectrum of patients was clinically expanded with the results from increased proportion of portal vein embolization and adjuvant therapy. In addition, ratio of Bismuth type III-IV (83.3% vs. 87.3%) in the preoperative diagnosis was increased.
Preoperative serum bilirubin levels, which are known to reflect liver status and surgical extension,8 were also an important factor for OS in this study; however, there was no significant difference in bilirubin levels in the between-period comparison. The presence of lymph node metastasis is an important independent predictor of long- term survival,5,22 although it showed no statistical significance. However, among the TNM stages, which are extended concept including tumor extent and lymph node metastasis, advanced stage (III and IV) was an important prognostic factor.
In recent years, surgical treatment for hilar cholangiocarcinoma has been evolving steadily, with an expanded indication, decreased mortality, and increased survival. Previous studies have reported lymph node metastasis, histopathologic status, resection margin status, and adjuvant chemotherapy as important prognostic factors for resected hilar cholangiocarcinoma.22-25 Recently, studies on neoadjuvant therapy have been conducted as a bridge modality to improve the rate of radical resection of locally advanced hilar cholangiocarcinoma.26-28 In view of these trends, especially those from 2000, the impact of surgical skills and methods remains important; however, the perioperative support and management are also thought to contribute to the oncological outcomes in patients with hilar cholangiocarcinoma.
In conclusion, the surgical and oncological outcomes have improved in patients with hilar cholangiocarcinoma, and the survival rate is expected to improve in the coming years, including that of patients with radical and concomitant vascular resections. The liver status, predicted by preoperative serum bilirubin levels, and advanced tumor stage, including tumor extension, are associated with poor prognosis in patients with hilar cholangiocarcinoma. However, there is limited evidence to accurately predict the prognosis with the currently known factors, and it is important to select a surgical candidate in consideration of the preoperative liver status and tumor extension.
REFERENCES
1. Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. 1999; Extended resections for hilar cholangiocarcinoma. Ann Surg. 230:808–818. discussion 819DOI: 10.1097/00000658-199912000-00010. PMID: 10615936. PMCID: PMC1420945.



2. Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, et al. 1998; Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 187:358–364. DOI: 10.1016/S1072-7515(98)00207-5. PMID: 9783781. PMCID: PMC2991118.



3. Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, Kondo S, et al. 1998; Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 5:52–61. DOI: 10.1007/PL00009951. PMID: 9683755.


4. Mizuno T, Ebata T, Nagino M. 2020; Advanced hilar cholangiocarcinoma: an aggressive surgical approach for the treatment of advanced hilar cholangiocarcinoma: perioperative management, extended procedures, and multidisciplinary approaches. Surg Oncol. 33:201–206. DOI: 10.1016/j.suronc.2019.07.002. PMID: 31301935.


5. Kang MJ, Jang JY, Chang J, Shin YC, Lee D, Kim HB, et al. 2016; Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg. 40:2451–2459. DOI: 10.1007/s00268-016-3551-9. PMID: 27206402.


6. Tran TB, Ethun CG, Pawlik TM, Schmidt C, Beal EW, Fields RC, et al. 2019; Actual 5-year survivors after surgical resection of hilar cholangiocarcinoma. Ann Surg Oncol. 26:611–618. DOI: 10.1245/s10434-018-7075-4. PMID: 30539494.


7. Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. 1997; Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 15:947–954. DOI: 10.1200/JCO.1997.15.3.947. PMID: 9060532.


8. Su CH, Tsay SH, Wu CC, Shyr YM, King KL, Lee CH, et al. 1996; Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 223:384–394. DOI: 10.1097/00000658-199604000-00007. PMID: 8633917. PMCID: PMC1235134.



9. Liu CL, Fan ST, Lo CM, Tso WK, Lam CM, Wong J. 2006; Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 93:1488–1494. DOI: 10.1002/bjs.5482. PMID: 17048280.


10. Han SS, Jang JY, Lee KU, Kim SW. 2008; Actual long-term outcome of Klatskin's tumor after surgical resection. Hepatogastroenterology. 55:1986–1992. PMID: 19260464.

11. Bismuth H, Nakache R, Diamond T. 1992; Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 215:31–38. DOI: 10.1097/00000658-199201000-00005. PMID: 1309988. PMCID: PMC1242367.



12. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. 2009; The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 250:187–196. DOI: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912.

13. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. 2011; Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 149:713–724. DOI: 10.1016/j.surg.2010.10.001. PMID: 21236455.


14. Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. 2012; Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 147:26–34. DOI: 10.1001/archsurg.2011.771. PMID: 22250108.

15. Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. 2013; Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 258:129–140. DOI: 10.1097/SLA.0b013e3182708b57. PMID: 23059502.

16. Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, et al. 2010; Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 17:476–489. DOI: 10.1007/s00534-009-0204-5. PMID: 19851704.


17. Furusawa N, Kobayashi A, Yokoyama T, Shimizu A, Motoyama H, Miyagawa S. 2014; Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 38:1164–1176. DOI: 10.1007/s00268-013-2394-x. PMID: 24305942.


18. Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. 2005; One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 137:148–155. DOI: 10.1016/j.surg.2004.06.006. PMID: 15674194.


19. Hwang HK, Jung MJ, Lee SH, Kang CM, Lee WJ. 2016; Adverse oncologic effects of intraoperative transfusion during pancreatectomy for left-sided pancreatic cancer: the need for strict transfusion policy. J Hepatobiliary Pancreat Sci. 23:497–507. DOI: 10.1002/jhbp.368. PMID: 27295957.


20. Kimura N, Toyoki Y, Ishido K, Kudo D, Yakoshi Y, Tsutsumi S, et al. 2015; Perioperative blood transfusion as a poor prognostic factor after aggressive surgical resection for hilar cholangiocarcinoma. J Gastrointest Surg. 19:866–879. DOI: 10.1007/s11605-014-2741-8. PMID: 25605416. PMCID: PMC4412428.



21. Young AL, Igami T, Senda Y, Adair R, Farid S, Toogood GJ, et al. 2011; Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB (Oxford). 13:483–493. DOI: 10.1111/j.1477-2574.2011.00328.x. PMID: 21689232. PMCID: PMC3133715.



22. Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. 2005; Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 241:77–84. DOI: 10.1097/01.sla.0000150166.94732.88. PMID: 15621994. PMCID: PMC1356849.



23. Capussotti L, Muratore A, Polastri R, Ferrero A, Massucco P. 2002; Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg. 195:641–647. DOI: 10.1016/S1072-7515(02)01481-3. PMID: 12437251.


24. Pichlmayr R, Weimann A, Klempnauer J, Oldhafer KJ, Maschek H, Tusch G, et al. 1996; Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 224:628–638. DOI: 10.1097/00000658-199611000-00007. PMID: 8916878. PMCID: PMC1235440.
25. Todoroki T, Kawamoto T, Koike N, Takahashi H, Yoshida S, Kashiwagi H, et al. 2000; Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 87:306–313. DOI: 10.1046/j.1365-2168.2000.01343.x. PMID: 10718799.


26. Jung JH, Lee HJ, Lee HS, Jo JH, Cho IR, Chung MJ, et al. 2017; Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma. World J Gastroenterol. 23:3301–3308. DOI: 10.3748/wjg.v23.i18.3301. PMID: 28566890. PMCID: PMC5434436.



27. Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, et al. 2018; Chemoradiotherapy for initially unresectable locally advanced cholangiocarcinoma. World J Surg. 42:2910–2918. DOI: 10.1007/s00268-018-4558-1. PMID: 29511872.


28. Katayose Y, Nakagawa K, Yoshida H, Morikawa T, Hayashi H, Okada T, et al. 2015; Neoadjuvant chemoradiation therapy for cholangiocarcinoma to improve R0 resection rate: the first report of phase II study. J Clin Oncol. 33(3 Suppl):402. DOI: 10.1200/jco.2015.33.3_suppl.402.

FIGURE AND TABLES
Table 1
Comparison of the adjuvant chemotherapy regimens between the two groups (period I and period II)
Table 2
Comparison of the clinical characteristics between the two groups (period I and period II)
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γGT, gamma glutamyl transferase; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen; PTBD, percutaneous transhepatic biliary drainage; ERCP, endoscopic retrograde cholangiopancreatography; ERBD, endoscopic retrograde biliary drainage; ENBD, endoscopic nasobiliary drainage
Table 3
Comparison of the type of surgery between the two groups (period I and period II)
Table 4
Comparison of the pathologic characteristics between the two groups (period I and period II)
Table 5
Comparison of the perioperative outcomes between the two groups (period I and period II)
Table 6
Univariate and multivariate regression analyses of prognostic factors for overall survival




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download