Abstract
Resection of the hepatic segments I+IV (S1+S4) is the most common type of parenchyma-preserving hepatectomy (PPH) for perihilar cholangiocarcinoma (PHCC). The author describes personal experience on the standard and modified techniques for PPH focused on S1+S4 resection in patients with PHCC. 1) Isolated caudate lobectomy with bile duct resection (BDR) is the minimal type of PPH, but not currently recommended due to technical difficulty. 2) Partial hepatectomy of S1+S4a±segment V (S5) with BDR provides wide operative field, but extension of BDR is limited and resection of S1 paracaval portion is still difficult. 3) Resection of S1+S4+S5 with BDR provides wider operative field for complete S1 resection and multiple biliary reconstruction. 4) Resection of S1+S4 with BDR offers very wide operative field and allows wider extent of hilar BDR, and thus presents the most common type of PPH. A supplementary video clip presents the detailed standard surgical procedure for resection of S1+S4 with BDR in a patient with type IIIA PHCC. 5) Modified resection of S1+S4±S5 or segment VIII (S8) with BDR facilitates additional resection of tumor-involved S5 or S8 ducts. 6) Major hilar vascular invasion is usually contraindicated for PPH and only small portal vein invasion requiring wedge resection and patch venoplasty is allowed. In conclusion, PPH can achieve curative resection and improved outcomes in patients with PHCC via reasonable modification of the extent of hepatectomy and hilar BDR. PPH may have advantages in selected patients depending on the extent of tumor, and in patients with high operative risk.
Extended hepatectomy enhances the surgical curability through obtaining tumor-free bile duct resection margins (BDRMs) compared with local resection in patients with perihilar cholangiocarcinoma (PHCC).1-7 However, extended hepatectomy can increase postoperative morbidity and mortality because it often entails massive liver resection which is strongly associated with post-hepatectomy liver failure (PHLF) and hepatic decompensation.8-10 Thus, parenchyma-preserving hepatectomy (PPH) can be considered as an alternative hepatectomy if extended hepatectomy carries a high risk of PHLF.10 Resection of the hepatic segments I+IV (S1+S4) is the most common type of PPH for PHCC. PPH has been usually indicated for selected patients in whom curative resection is expected through PPH because PPH may result in lower surgical curability compared with extended hepatectomy. However, in practice, the decision to determine surgical curability for PHCC is often difficult before or even during surgery. The cumulative experience involving PHCC has led to the expansion of indications for PPH because the extent of hilar bile duct resection (BDR) can be increased toward the left, right or both liver sides in order to obtain tumor-free BDRMs.11 The author describes personal experience with the standard and modified techniques for PPH focused on S1+S4 resection in patients with PHCC.
Considering the tumor invasion process in PHCC, it is mandatory to resect the caudate lobe (S1) in combination with various extents of hepatectomy.3 The author previously reported isolated S1 resection with BDR in a patient with Bismuth-Corlette type IV PHCC.12 The operative field during and after isolated S1 resection was very narrow, thus multiple hepaticojejunostomies for the 3 right and 4 left hepatic duct openings was very demanding (Fig. 1). Because of such technical challenges and the high risk of biliary reconstruction-associated complications, this technique was no longer recommended.
To address the technical difficulty encountered during isolated S1 resection with BDR, the ventral portion of segment IV (S4a) with or without the ventral portion of segment V (S5) was concurrently resected. Such partial resection of S1+S4a±S5 widened the operative field, which facilitated S1 resection and multiple biliary reconstruction (Fig. 2). However, it is usually indicated only for cases involving type I or II PHCC due to the limited extension of the BDRMs and difficult resection of the S1 paracaval portion.
S1+S4a+S5 resection along with BDR facilitates complete S1 resection and biliary reconstruction via wide operative field.13 This technique entails transection of the middle hepatic vein (MHV), and thus designated as Taj Mahal resection due to the shape of hepatic resection. However, it induced detrimental hepatic venous congestion at the MHV drainage territory in the right anterior section. To prevent such unnecessary hepatic venous congestion, the author reported S1+S4a+S5 resection with BDR after preservation of the MHV trunk (Fig. 3).14 This procedure yielded wider operative field for complete S1 resection and multiple biliary reconstructions.
Complete transection of the liver into right and left livers provides very wide operative field and allows wider hilar BDR, which is an advantage for complete S1 resection and multiple biliary reconstruction; it also enables expansion of the extent of BDRMs deep into the right and left liver sides. Resection of the S1+S4 is the simplest and most effective PPH (Fig. 4).15 The extent of hilar BDR is compatible with that of right trisectionectomy on the side of left hepatic duct and that of left hepatectomy toward the right hepatic duct. Due to very wide operative field, the procedure is not contraindicated by anatomical variation (Fig. 5A) or focal tumor invasion of the hilar portal vein (Fig. 5B).
Depending on the extent of tumor invasion, the extent of S1+S4 resection can be non-anatomically expanded to obtain tumor-free BDRMs. When the right anterior or posterior bile duct was extensively involved, the medial part of the right anterior section was further transected to remove the involved segment V ducts (B5) or segment VIII ducts (B8s) (Fig. 6).12 The middle hepatic vein trunk can also be resected to match the additional partial resection of the right anterior section (Fig. 6).
Major vascular invasion involving the hepatic artery or portal vein warranting segmental resection and anastomosis is an established contraindication for PPH because such vascular reconstruction makes PPH much more difficult and complex than extended hepatectomy. Concurrent resection of major vascular invasion has been rarely performed during PPH (Fig. 7), but it is strongly contraindicated because of the complexity of the surgical procedures and disappointing postoperative outcomes. Conversely, small-sized portal vein invasion requiring only wedge resection and patch venoplasty (Fig. 7B) is permissible for PPH because such portal vein invasion is usually not detected in the preoperative imaging studies.
A 75-year-old male patient with obstructive jaundice was admitted with a diagnosis of PHCC. Preoperative imaging studies revealed that the tumor was compatible with type IIIA PHCC without vascular invasion (Fig. 8A-C). Obstructive jaundice resolved slowly with repeated episodes of cholangitis despite external biliary drainage, thus the operation was performed after biliary decompression at a serum total bilirubin concentration of 3.4 mg/dl. Considering the old age and unresolved jaundice in the patient, the author decided to perform S1+S4 resection with BDR.
After laparotomy, surgical resectability was assessed by manual palpation. The distal common bile duct was dissected first and the status of tumor invasion was assessed to determine tumor-free distal BDRM in intraoperative frozen-section biopsy. Dissection of the hepatoduodenal ligament was continued toward the hepatic hilum, and the hepatic artery and portal vein branches were successfully isolated (Fig. 9A).
The liver surface was marked to define the territory of hepatic resection (Fig. 9B). The liver parenchyma was transected along the falciform ligament. The left portal vein and hepatic artery were further dissected to expose the umbilical portion of the left portal vein. The segment IV duct (B4), segment III duct (B3) and segment II duct (B2) were consecutively transected (Fig. 9C), ensuring tumor-free BDRMs. Hepatic parenchymal transection continued toward the dorsal portion of the left caudate lobe, and the remnant left lateral section was completely separated from the left caudate lobe (Fig. 9D).
The right-sided hepatic parenchymal transection was performed along the hemi-liver discoloration line (Fig. 10A). At the right hepatic hilum, two segment V ducts (B5s) and two segment VIII ducts (B8s) were transected (Fig. 10B), and were found tumor-free. The right posterior duct, a conjoined portion of the segment VI and VII ducts (B6+7) was transected, which was also tumor-free. The paracaval portion of S1 combined with the Spigelian lobe was removed along with S4 resection (Fig. 10C, D).
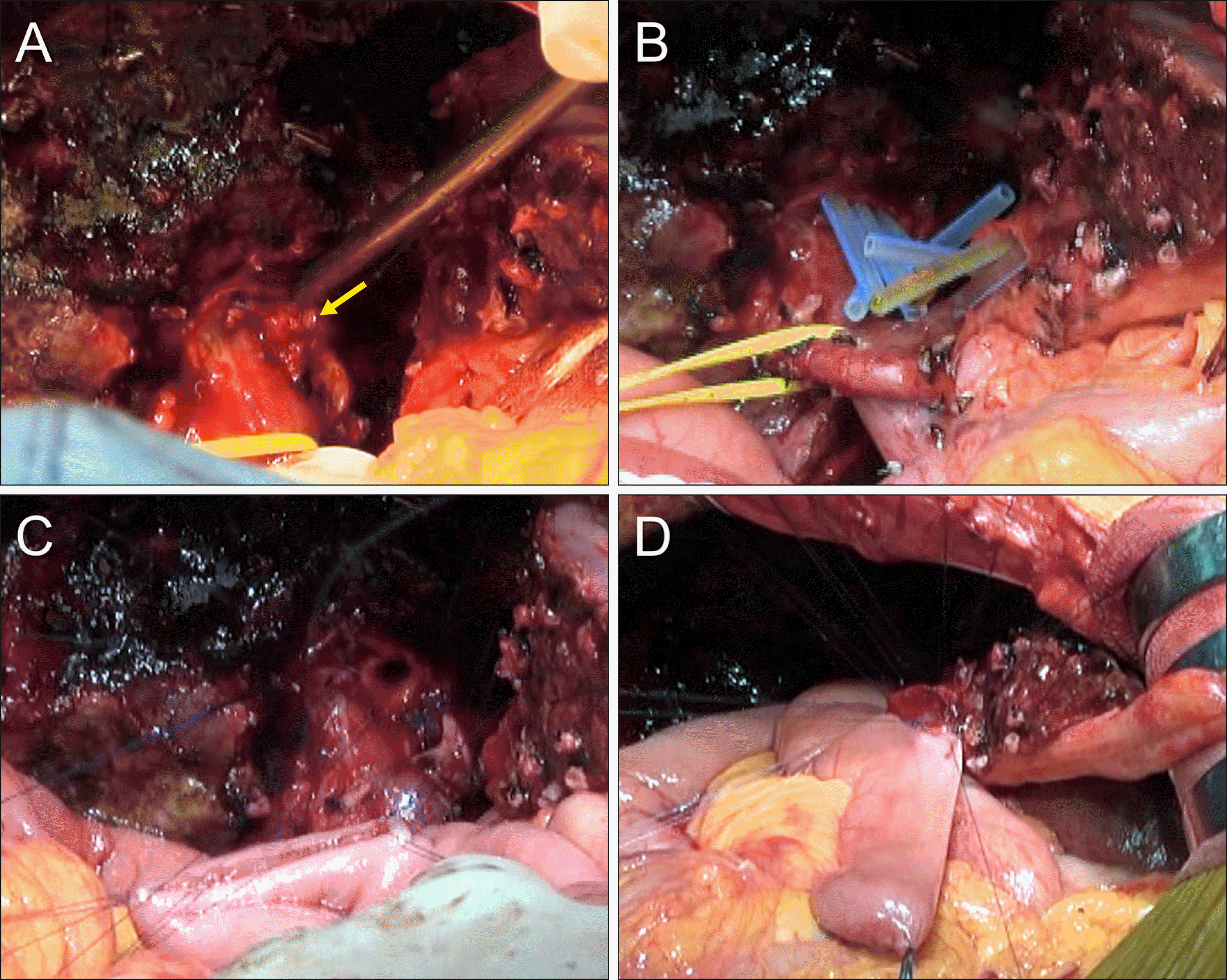
The two B5s and two B8s openings were placed within the common connective tissue sheath, which was separated from a single B6+7 opening. Thus, unification ductoplasty was performed to conjoin these right-sided ducts (Fig. 11A). The 4-cm-wide conjoined right hepatic duct was reconstructed through a single hepaticojejunostomy (Fig. 11B, C). The three hepatic duct openings at the left lateral section (one B2, one B3 and one remnant B4) were also within the common connective tissue sheath, thus obviating the need for ductoplasty. A single hepaticojejunostomy was performed at the remnant left lateral section ducts (Fig. 11B, D). To manipulate the jejunal limb freely without tension during hepaticojejunostomy, a distance of 8 cm was ensured at the jejunal limb between the right- and the left-sided hepaticojejunostomies. The operation took 6 hours. Six sessions of intraoperative frozen-section biopsy for DBRMs were performed. The detailed surgical procedures are presented in an 8-minutes supplementary video clip (Supplementary Video).
The pathology report revealed a 4.0 cm-sized cholangiocarcinoma which was well differentiated and extended beyond the bile duct to a depth of 6 mm from the surface epithelia. There was perineural invasion without lymphovascular invasion. Metastasis involving 3 of 11 lymph nodes was observed. The extent of the tumor was pT2aN1M0, and thus regarded as the stage IIIC according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system.
The patient recovered uneventfully (Fig. 8D) and was discharged from the hospital on the postoperative day 21. Because of poor general condition and patient’s refusal, the patient did not receive adjuvant chemotherapy. Multiple recurrences occurred 16 months after surgery, and the patient passed away 24 months after surgery due to tumor progression.
It is important to obtain tumor-free resection margin during the surgical treatment of PHCC.5,16 Extended hepatectomy has been preferred to achieve curative resection of PHCC because it facilitates tumor-free BDRMs and is also effective to manage major hilar vascular invasion. However, the majority of patients with PHCC manifest obstructive jaundice, which decreases the hepatic functional reserve, even after sufficient biliary decompression. Thus, extended hepatectomy can increase postoperative morbidity and mortality because it can result in PHLF, hepatic decompensation and secondary septic complications.8-10 To decrease the risk of PHLF by reducing the hepatic parenchymal resection rate, preoperative right portal vein embolization with or without hepatic vein embolization has been performed.17,18 The author has also performed left portal vein embolization in patients undergoing left hepatectomy with high operative risk.19
Hepatic resection, limited as much as possible to what is necessary for curative resection, might lead to fewer postoperative complications in patients with PHCC. It was suggested to perform limited hepatic resection according to the individual tumor extent of each patient.4 Because S1 resection is known to be a prerequisite for curative resection of PHCC,20 resection of the entire S1 can be the most limited PPH in surgical resection for PHCC.12 Various combinations of S1 resection with S4 and S5 resection are presented in the present study.13-15 Of them, S1+S4 resection appears to be the most reasonable extent of PPH for patients with PHCC after considering the complexity of surgical procedures and the extent of hilar BDR.15 The parenchymal resection rate through S1+S4 resection is estimated to be approximately 10%-15% of the whole liver volume.
No standardized surgical procedures of S1+S4 resection with BDR are reported in the literature. Based on the surgical techniques for PHCC in our institution, it is reasonable to perform hepatic transection along the plane of right trisectionectomy initially, which separates the left lateral section completely from the caudate lobe. It is usually not possible to further extend the left BDRM toward the left side because the B2 and B3 ducts are transected close to the umbilical portion of the left portal vein. Following the left-sided transection of the hepatic parenchyma, a clear hemihepatic discoloration appears at the remnant liver due to the complete occlusion of the inflow vessels to the S4. Subsequently, the classical left hepatectomy with caudate lobectomy is performed, in which the paracaval portion of S1 ventral to the middle hepatic vein trunk is completely removed. Unlike the left-sided hepatic ducts, the right-sided hepatic ducts can be transected after further resection of the right anterior section parenchyma to obtain tumor-free BDRMs. The maximal extent of additional right-sided hepatic resection is concurrent resection of the entire right anterior section parenchyma, which converts the hepatectomy into central bisectionectomy+S1 resection. Since such extensive hepatectomy is no longer regarded as PPH, it was not described in detail in the present study.
The clinical implications of PPH for PHCC have yet to be clearly described. It is a matter of concern that PPH decreases the rate of curative surgical resection, but several studies reported that surgical curability was not impaired by PPH and PPH elicited favorable postoperative prognosis similar to that of extended hepatectomy.10,15,21 Thus, PPH may reduce surgical morbidity and mortality associated with PHCC when it is utilized for carefully selected indication. Meanwhile, it is also reported that PPH was not suitable as an operative procedure to achieve curative resection in the majority of patients with PHCC. A Japanese study reported that only 15% of patients could be selected to undergo PPH as a limited hepatectomy procedure for achieving curative resection.10
In contrast to the conventional concept of S1+S4 resection with BDR, some patients with advanced tumors including type IIIA and IV PHCC have been treated with PPH, especially when either right or left hepatectomy may not ensure surgical curability. If preoperative right portal vein embolization is already performed and the left BDRM is tumor-positive, the surgery will be R1 resection unless right trisectionectomy is performed. If the right BDRMs are tumor-positive following left hepatectomy, it is also not easy to expand the extent of hilar BDR. Conversely, customized central hepatectomy in the form of S1+S4 resection can provide a chance to expand the extent of resection to obtain tumor-free BDRMs because it can facilitate additional resection toward the left, right or both liver sides.
S1+S4 resection with BDR entails more complex surgical procedures than extended hepatectomy and the operation time is usually 6-8 hours. When performing multiple bilio-enteric anastomoses, conjoined ductoplasty for unification of the adjacent bile duct openings can reduce the number of separate hepaticojejunostomy. Successful PPH requires comprehensive preoperative anatomic recognition, accurate evaluation of tumor extent, and skillful surgical techniques for meticulous hepatectomy and biliary reconstruction.10,17,22,23
In conclusion, PPH can achieve curative resection and improved outcomes in patients diagnosed with PHCC via reasonable modification of the extent of hepatectomy and hilar BDR. PPH may have advantages in the selected patients according to the extent of tumor, and in the patients with high operative risk.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.2021.25.1.112.
REFERENCES
1. Baer HU, Stain SC, Dennison AR, Eggers B, Blumgart LH. 1993; Improvements in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 217:20–27. DOI: 10.1097/00000658-199301000-00005. PMID: 8380975. PMCID: PMC1242729.



2. Childs T, Hart M. 1993; Aggressive surgical therapy for Klatskin tumors. Am J Surg. 165:554–557. DOI: 10.1016/S0002-9610(05)80433-7. PMID: 7683844.


3. Tsuzuki T, Ueda M, Kuramochi S, Iida S, Takahashi S, Iri H. 1990; Carcinoma of the main hepatic duct junction: indications, operative morbidity and mortality, and long-term survival. Surgery. 108:495–501. PMID: 2396193.

4. Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. 1990; Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 14:535–543. discussion 544DOI: 10.1007/BF01658686. PMID: 2166381.


5. Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, et al. 1998; Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 123:131–136. DOI: 10.1016/S0039-6060(98)70249-1. PMID: 9481397.


6. Iida S, Tsuzuki T, Ogata Y, Yoneyama K, Iri H, Watanabe K. 1987; The long-term survival of patients with carcinoma of the main hepatic duct junction. Cancer. 60:1612–1619. DOI: 10.1002/1097-0142(19871001)60:7<1612::AID-CNCR2820600732>3.0.CO;2-1. PMID: 3621131.


7. Hayashi S, Miyazaki M, Kondo Y, Nakajima N. 1994; Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer. 73:2922–2929. DOI: 10.1002/1097-0142(19940615)73:12<2922::AID-CNCR2820731208>3.0.CO;2-K. PMID: 8199989.


8. Blumgart LH, Hadjis NS, Benjamin IS, Beazley R. 1984; Surgical approaches to cholangiocarcinoma at confluence of hepatic ducts. Lancet. 1:66–70. DOI: 10.1016/S0140-6736(84)90002-3. PMID: 6197596.


9. Miyazaki M, Itoh H, Ambiru S, Shimizu H, Togawa A, Gohchi E, et al. 1996; Radical surgery for advanced gallbladder carcinoma. Br J Surg. 83:478–481. DOI: 10.1002/bjs.1800830413. PMID: 8665234.


10. Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Okaya T, et al. 1999; Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg. 189:575–583. DOI: 10.1016/S1072-7515(99)00219-7. PMID: 10589594.


11. Hwang S. Parenchyma-preserving hepatectomy including segment IV+I resection in a patient with type IV perihilar cholangiocarcinoma: A case report with video clip. [in press].

12. Hwang S, Ha TY, Kim JS, Kim KH, Lee SG, Lee YJ. 2003; Isolated caudate lobectomy with bile duct resection performed in a patient with type IV hilar bile duct cancer. J Korean Surg Soc. 64:441–446.
13. Kawarada Y, Isaji S, Taoka H, Tabata M, Das BC, Yokoi H. 1999; S4a+S5 with caudate lobe (S1) resection using the Taj Mahal liver parenchymal resection for carcinoma of the biliary tract. J Gastrointest Surg. 3:369–373. DOI: 10.1016/S1091-255X(99)80052-3. PMID: 10482688.

14. Hwang S, Moon DB, Park EH, Kim MH, Lee YJ, Lee SG. 2003; S4a+S5 with caudate lobe (S1) resection as a parenchyma-preserving liver resection for a patient with type IIIb hilar bile duct cancer. J Korean Surg Soc. 64:515–520.
15. Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Shimizu Y, et al. 1998; Segments I and IV resection as a new approach for hepatic hilar cholangiocarcinoma. Am J Surg. 175:229–231. DOI: 10.1016/S0002-9610(97)00295-X. PMID: 9560126.


16. Klempnauer J, Ridder GJ, Werner M, Weimann A, Pichlmayr R. 1997; What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer. 79:26–34. DOI: 10.1002/(SICI)1097-0142(19970101)79:1<26::AID-CNCR5>3.0.CO;2-K. PMID: 8988723.

17. Lee SG, Hwang S. 2005; How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 12:38–43. DOI: 10.1007/s00534-004-0949-9. PMID: 15754098.


18. Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, et al. 2015; Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg. 39:2990–2998. DOI: 10.1007/s00268-015-3194-2. PMID: 26304608.


19. Hwang S, Ko GY, Kim MH, Lee SK, Gwon DI, Ha TY, et al. 2016; Preoperative left portal vein embolization for left liver resection in high-risk hepatobiliary malignancy patients. World J Surg. 40:2758–2765. DOI: 10.1007/s00268-016-3618-7. PMID: 27384172.


20. Mizumoto R, Kawarada Y, Suzuki H. 1986; Surgical treatment of hilar carcinoma of the bile duct. Surg Gynecol Obstet. 162:153–158. PMID: 3945893.

21. Chen XP, Lau WY, Huang ZY, Zhang ZW, Chen YF, Zhang WG, et al. 2009; Extent of liver resection for hilar cholangiocarcinoma. Br J Surg. 96:1167–1175. DOI: 10.1002/bjs.6618. PMID: 19705374.


22. Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, et al. 2010; Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 17:476–489. DOI: 10.1007/s00534-009-0204-5. PMID: 19851704.


23. Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB, et al. 2015; Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg. 19:1305–1314. DOI: 10.1007/s11605-015-2846-8. PMID: 25947549.


FIGURES
Fig. 1
Perioperative findings of isolated caudate lobectomy and bile duct resection. (A, B) Preoperative magnetic resonance and direct tube cholangiography images show type IV perihilar cholangiocarcinoma. Circles indicate the individual locations of bile duct transection. (C, D) Postoperative magnetic resonance images taken 4 weeks after surgery show the uneventful status of multiple hepaticojeunostomies and the extent of hepatic parenchymal resection.
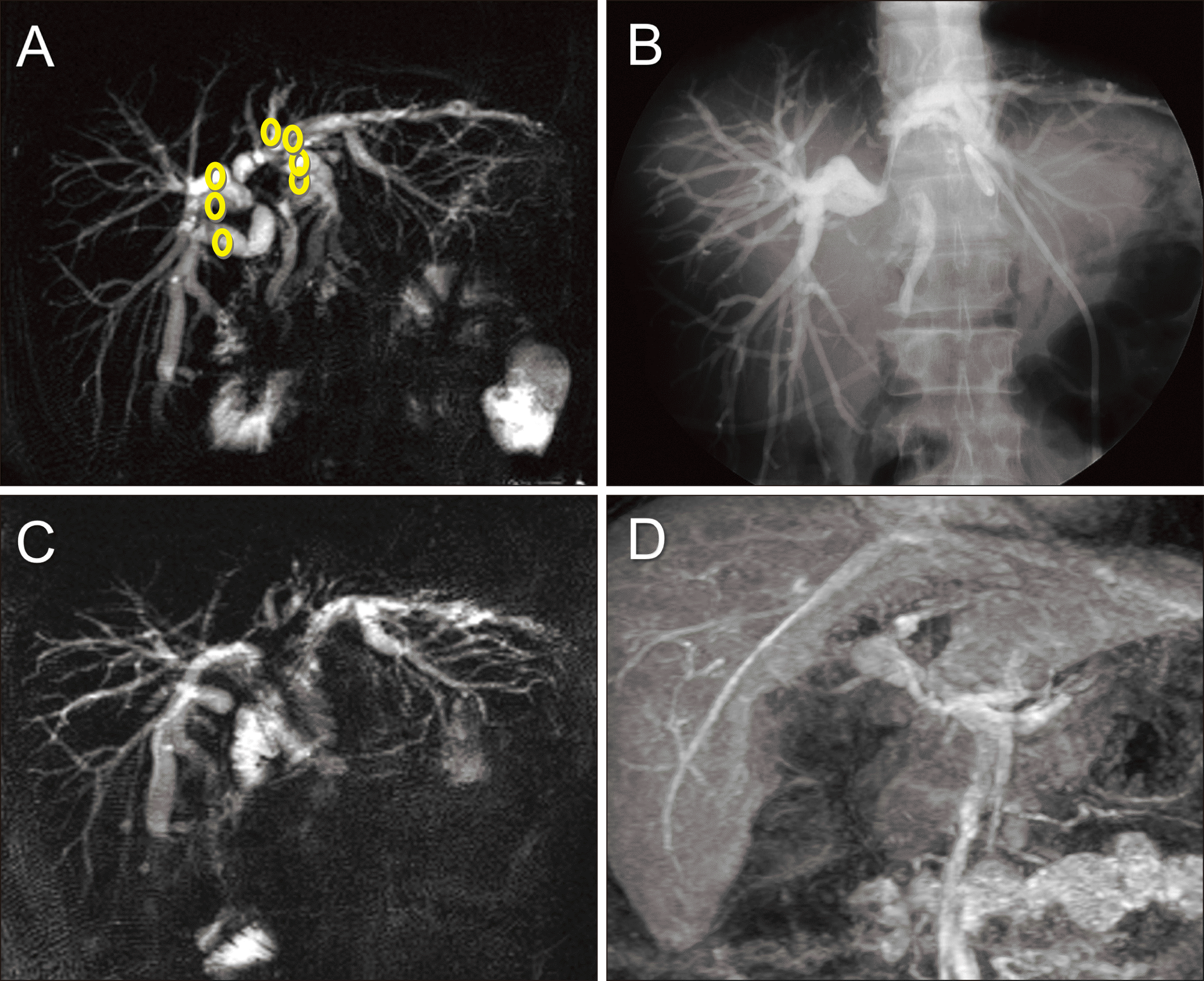
Fig. 2
Intraoperative Intraoperative photographs showing resection of the segments I+IV+V. (A, B) The segments IV and V are partially resected, and then the paracaval portion and Spigelian lobe of the caudate lobe are resected partially in a patient with type II perihilar cholangiocarcinoma. Multiple bile duct openings are exposed after hilar bile duct resection. (C, D) In another patient with type I perihilar cholangiocarcinoma, the segments IV and V are partially resected concurrently with the Spigelian lobe. The shape of the resected specimen indicates the extent of surgical resection.
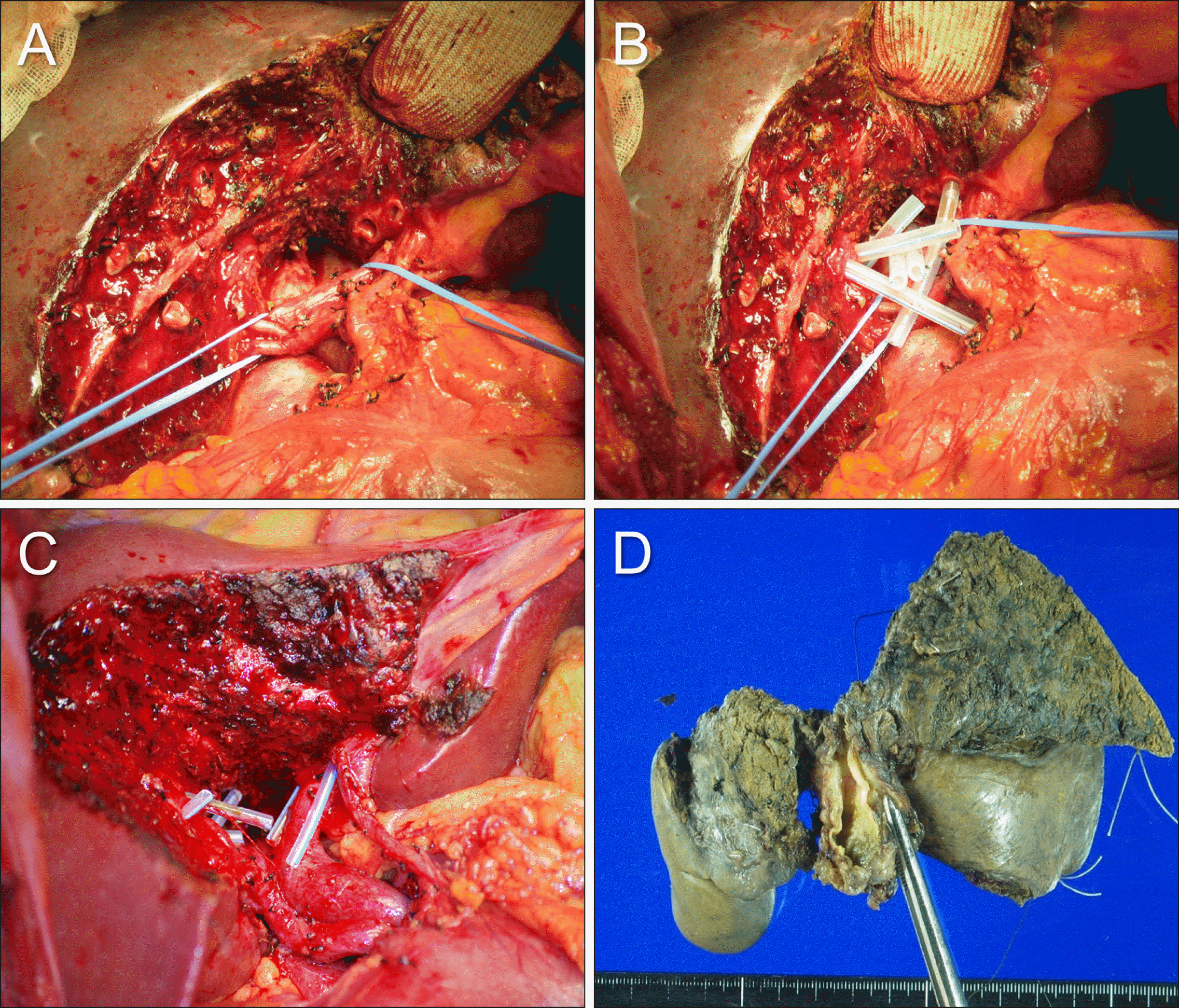
Fig. 3
Intraoperative photographs showing the extent of hepatectomy following resection of the segments I+IVa+V in a patient with type IIIB perihilar cholangiocarcinoma. (A) The hepatoduodenal ligament was skeletonized. (B) Resection of the segments I+IVa+V is performed with preservation of the middle hepatic vein trunk. Silastic stents are inserted in the multiple bile duct openings. (C) The resected specimen is visible, indicating the extent of surgical resection. (D) Single hepaticojejunstomy involves each of the right- and left-sided conjoined ducts.
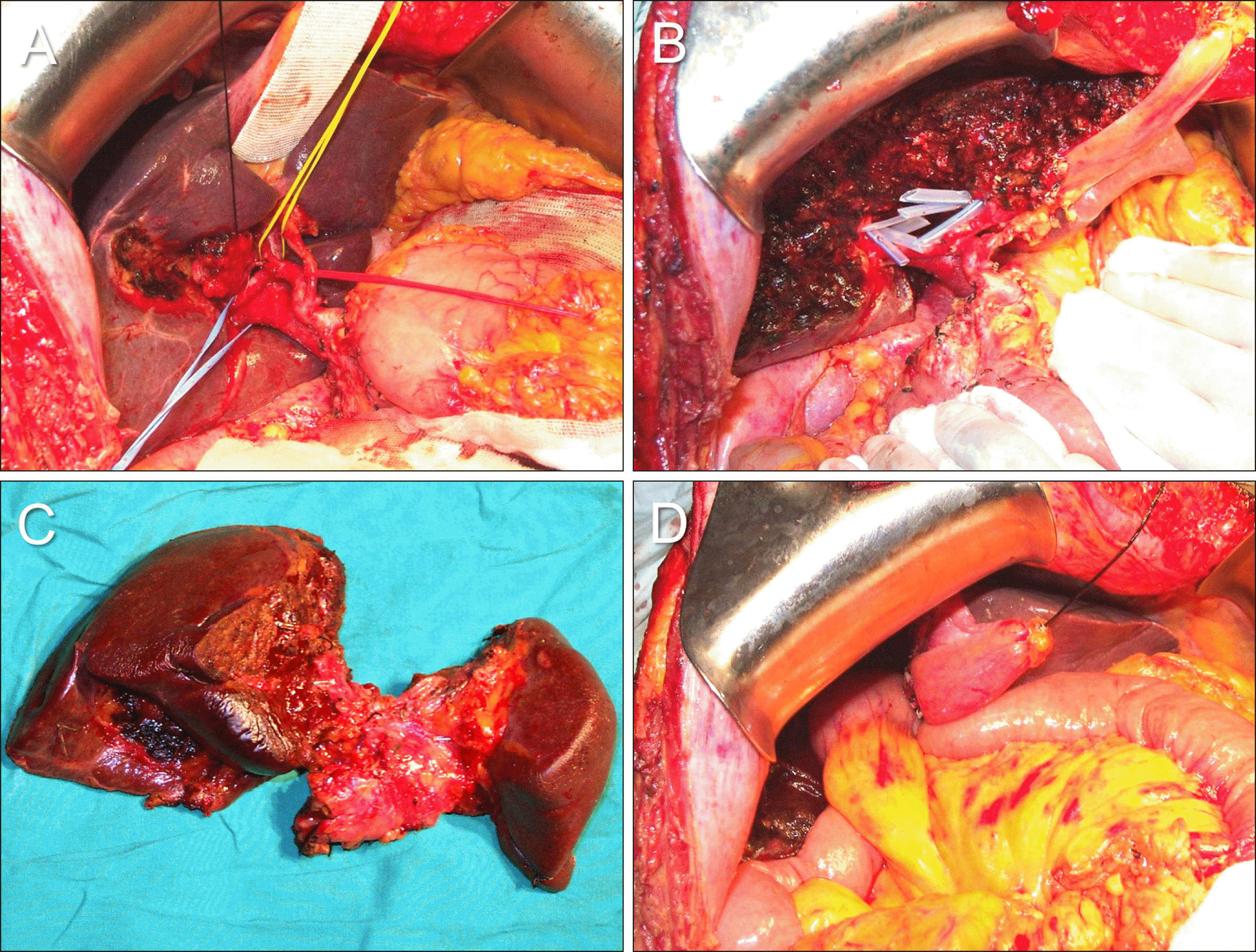
Fig. 4
Intraoperative photographs showing the extent of hepatectomy following resection of the segments I+IV in a patient with type II perihilar cholangiocarcinoma. (A) The extent of segment IV resection is marked at the liver surface. (B) The surgical specimen shows resection of the segments I+IV and bile duct resection. The middle hepatic vein trunk is completely preserved with excavation of the paracaval portion of the caudate lobe. (C) Two hepaticojejunstomies involving the right-sided conjoined ducts and one involving the left- sided conjoined ducts are shown.
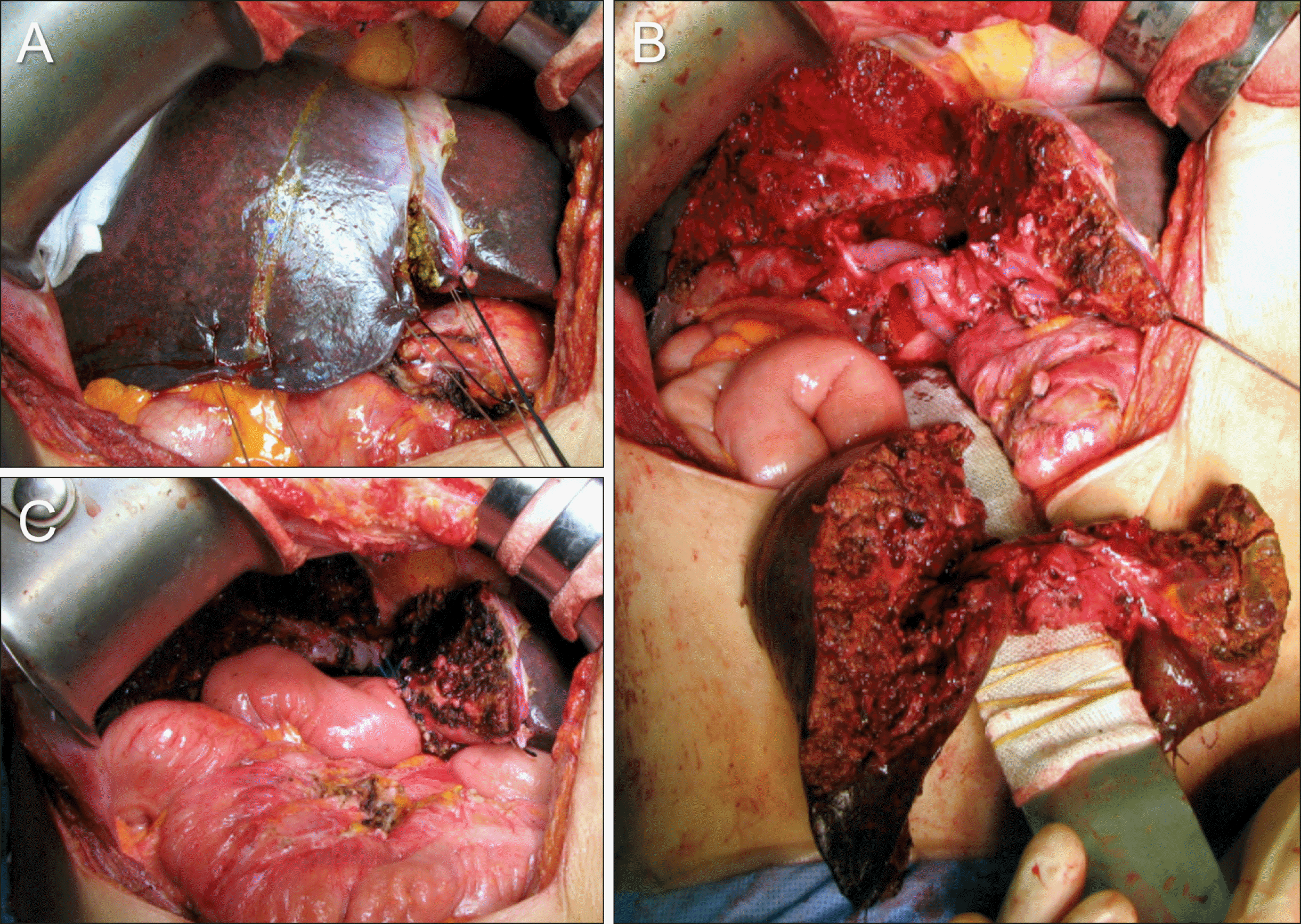
Fig. 5
Intraoperative photographs showing anatomical variations observed during resection of the segments I+IV. (A) Type III portal vein anomaly is visible. (B) Early branching of the segment V duct (B5) is visible (arrow). This anatomy of the right hepatic duct required three separate hepaticojejunostomies, each to the B5, segment VIII duct (B8) and segments VI+VII duct (B6+7). (C) The ventral end of the middle hepatic vein trunk is not dissected to protect the fissural vein at the remnant segment IV parenchyma. (D) The middle hepatic vein trunk is transected to remove the paracaval portion of the caudate lobe completely.
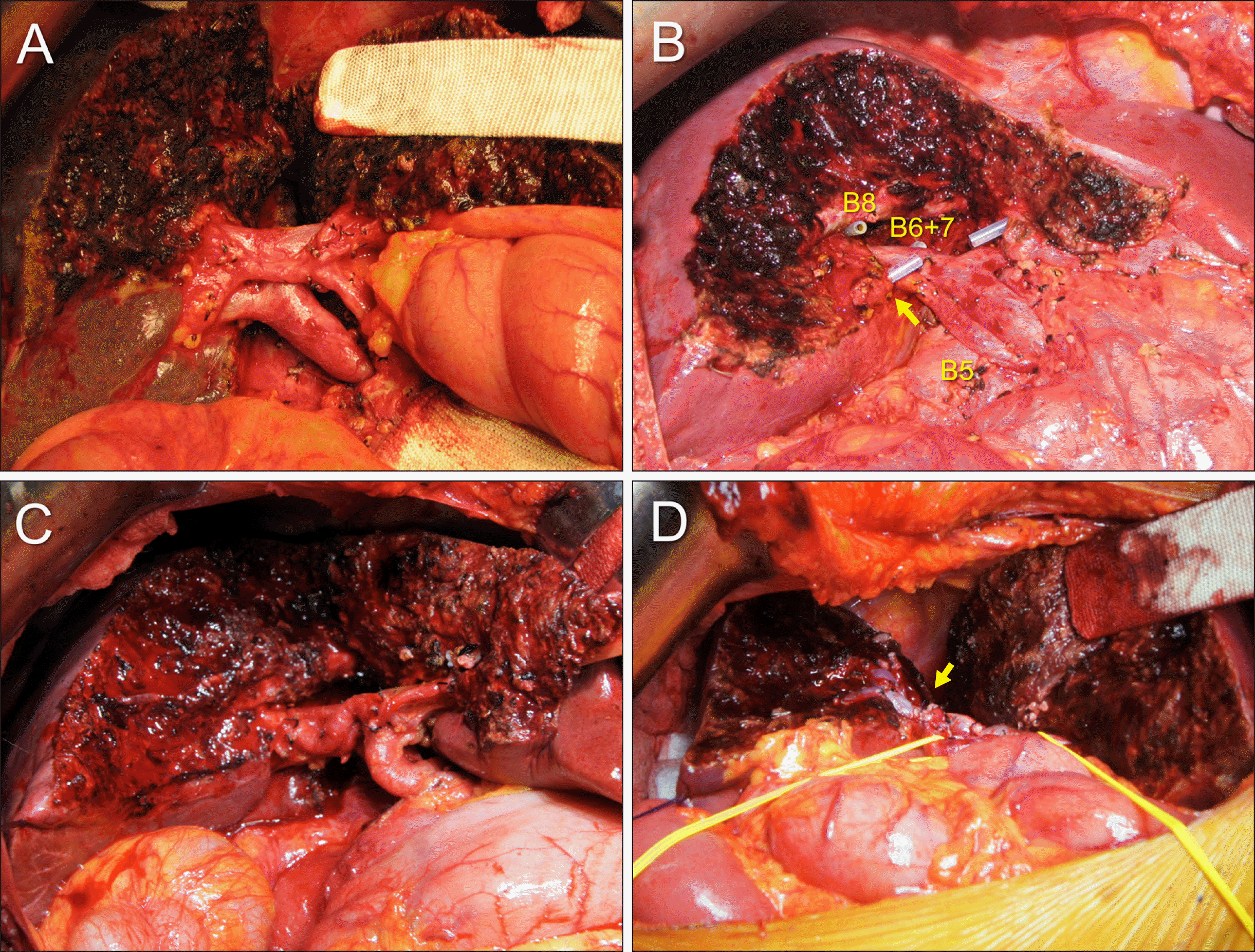
Fig. 6
Intraoperative photographs showing additional resection to the usual segments I+IV resection. (A) One branch of the segment V ducts is resected with the corresponding hepatic parenchyma. An arrow indicates the resected branch stump. (B) One branch of the segment VIII ducts is resected with the corresponding hepatic parenchyma (arrow). (C) The medial part of the right anterior section is resected with exposure of the right hepatic vein (arrow). (D) Most of the right anterior section is resected along with exposure of the right hepatic vein (arrow).
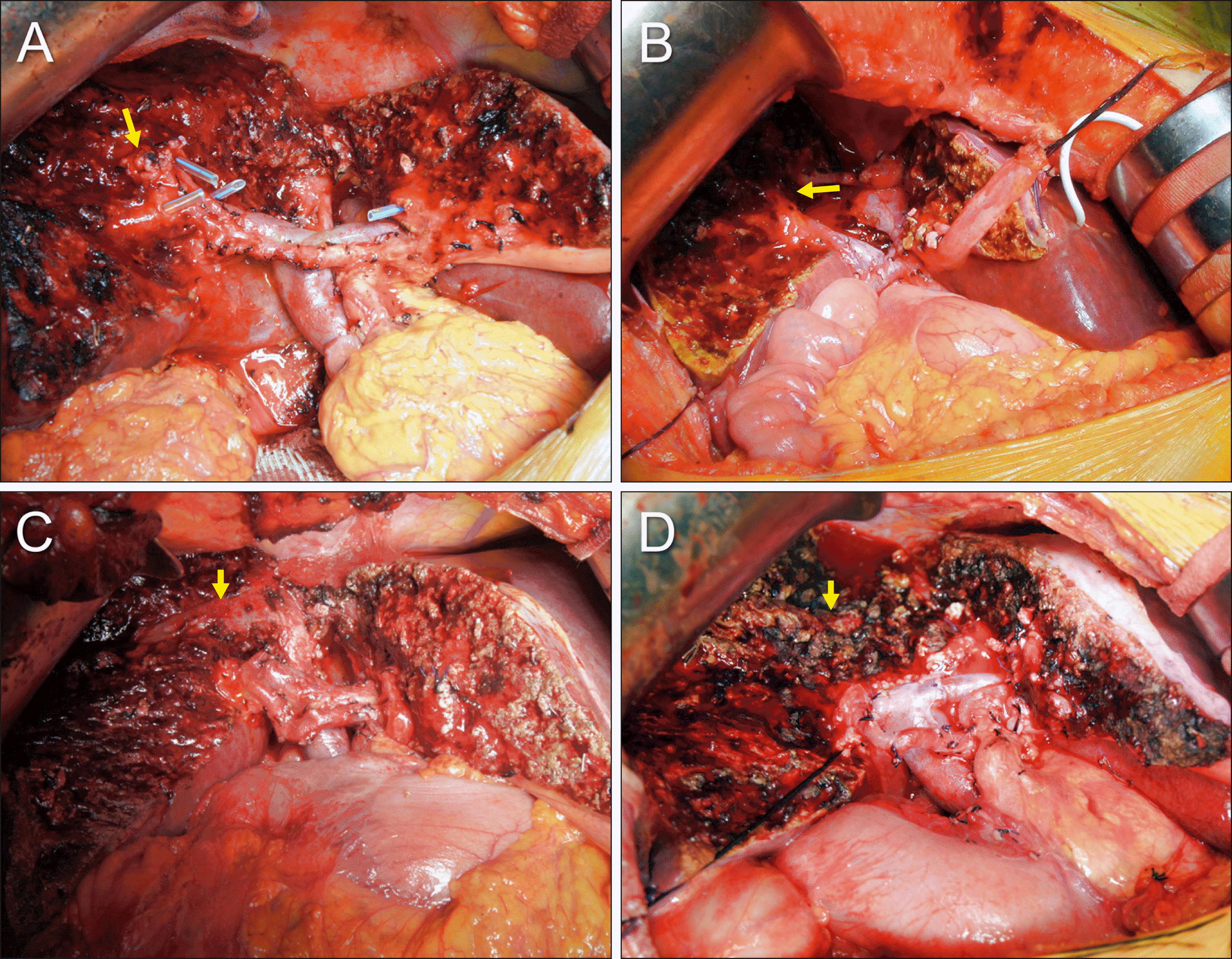
Fig. 7
Intraoperative photographs showing vascular reconstruction combined with resection of the segments I+IV. (A, B) The left portal vein is invaded by the tumor, warranting wedge resection and venoplasty using an iliac vein allograft patch. (C) An iliac vein allograft (arrow) is interposed at the left portal vein. (D) The right hepatic artery is interposed with an autologous greater saphenous vein graft conduit (bidirectional arrow).
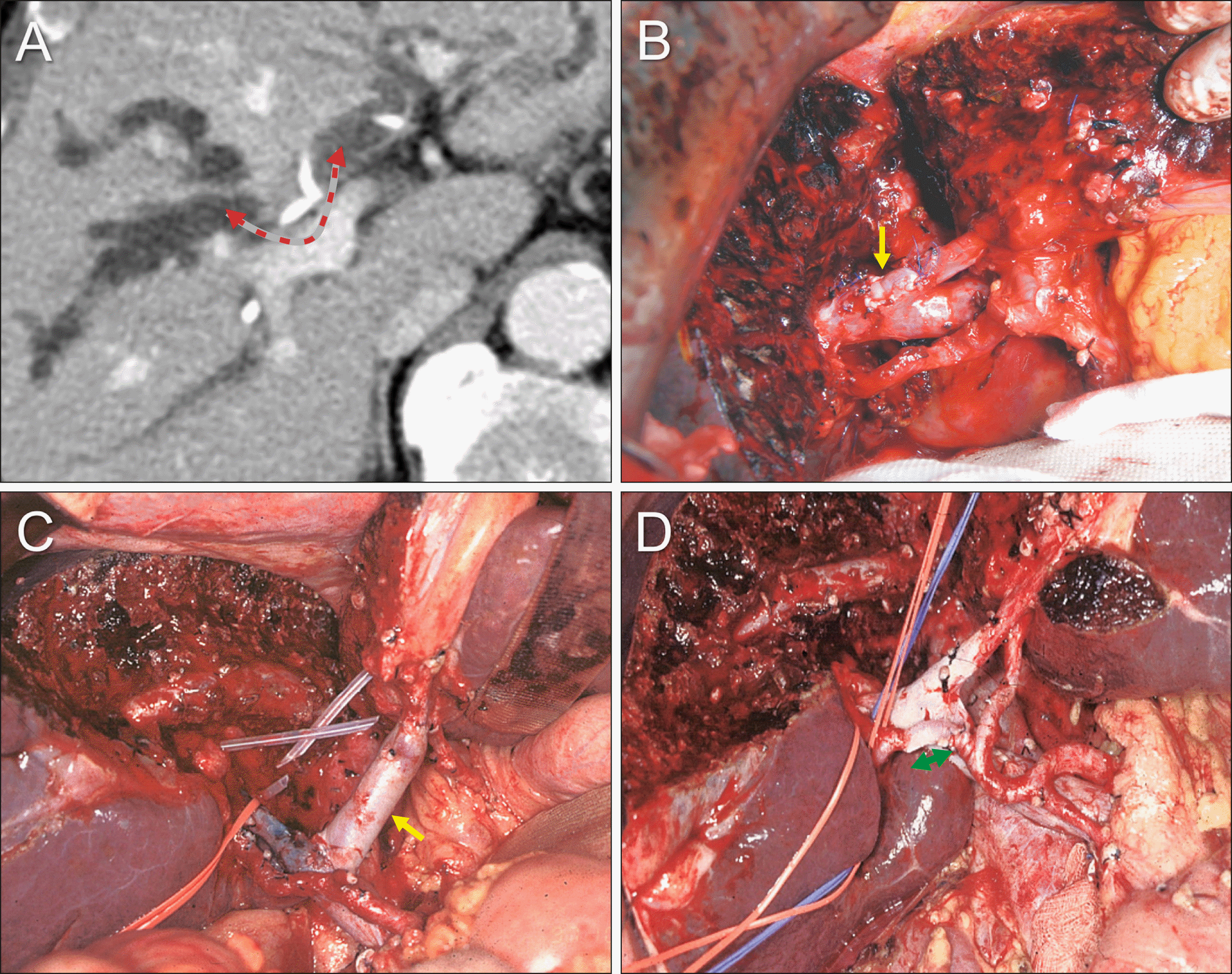
Fig. 8
Perioperative findings of segments I+IV resection and bile duct resection in a 75-year-old male patient with type IIIA perihilar cholangiocarcinoma. (A, B) Preoperative computed tomography and magnetic resonance cholangiography taken before biliary decompression show marked dilatation of all intrahepatic ducts. (C) Preoperative computed tomography taken after biliary decompression still reveals marked dilatation of the intrahepatic ducts. (D) Computed tomography taken 2 weeks after resection of segments I+IV shows no abnormal findings.
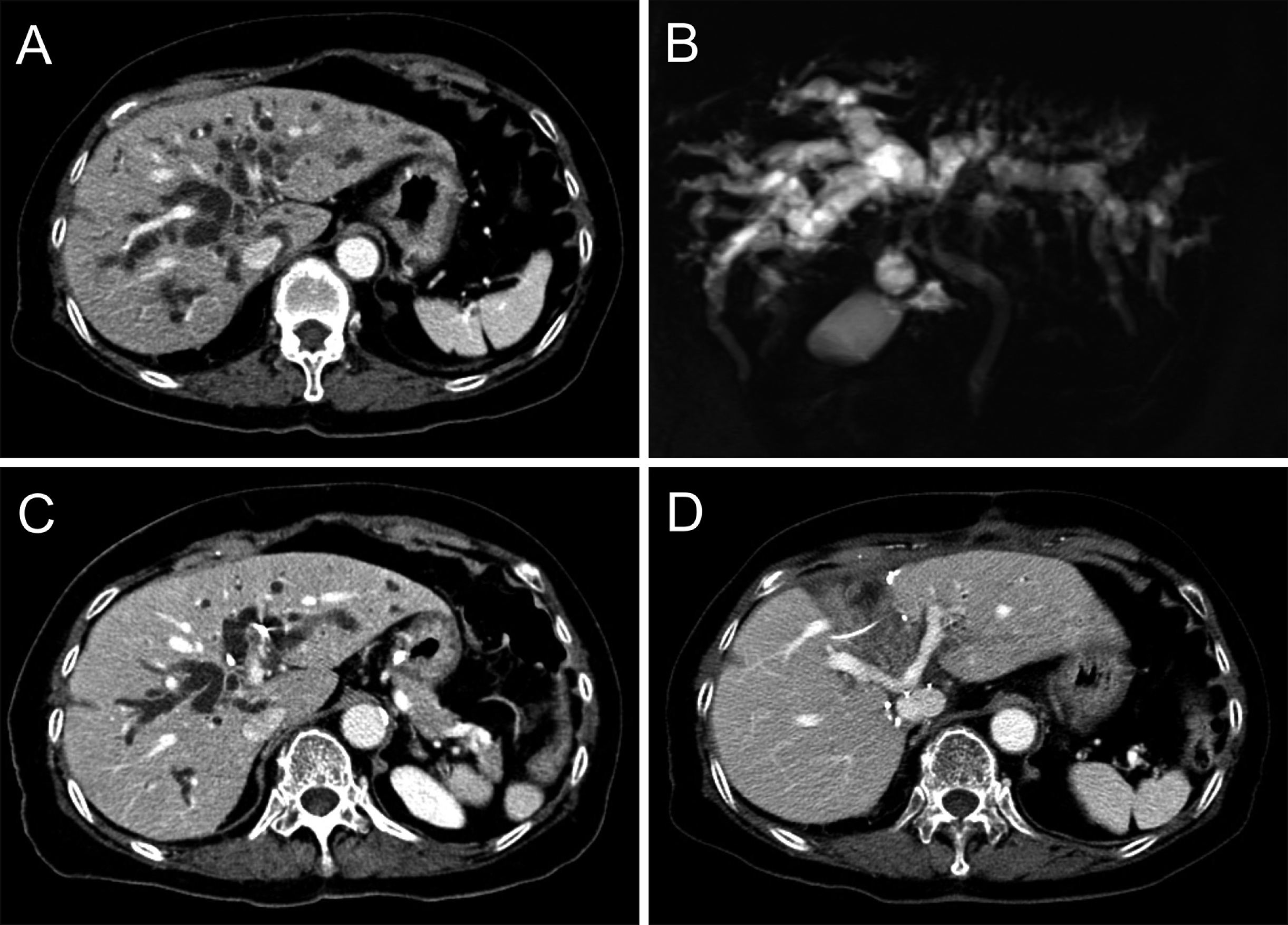
Fig. 9
Intraoperative photographs showing the surgical procedure for resection of segments I+IV and bile duct. (A) The hepatic artery is encircled after transection of the distal bile duct. (B) The liver surface is marked to define the territory of hepatic resection. (C) The liver parenchyma was transected along the falciform ligament and the left lateral section ducts are transected (arrow). (D) The remnant left lateral section is completely separated from the left caudate lobe.
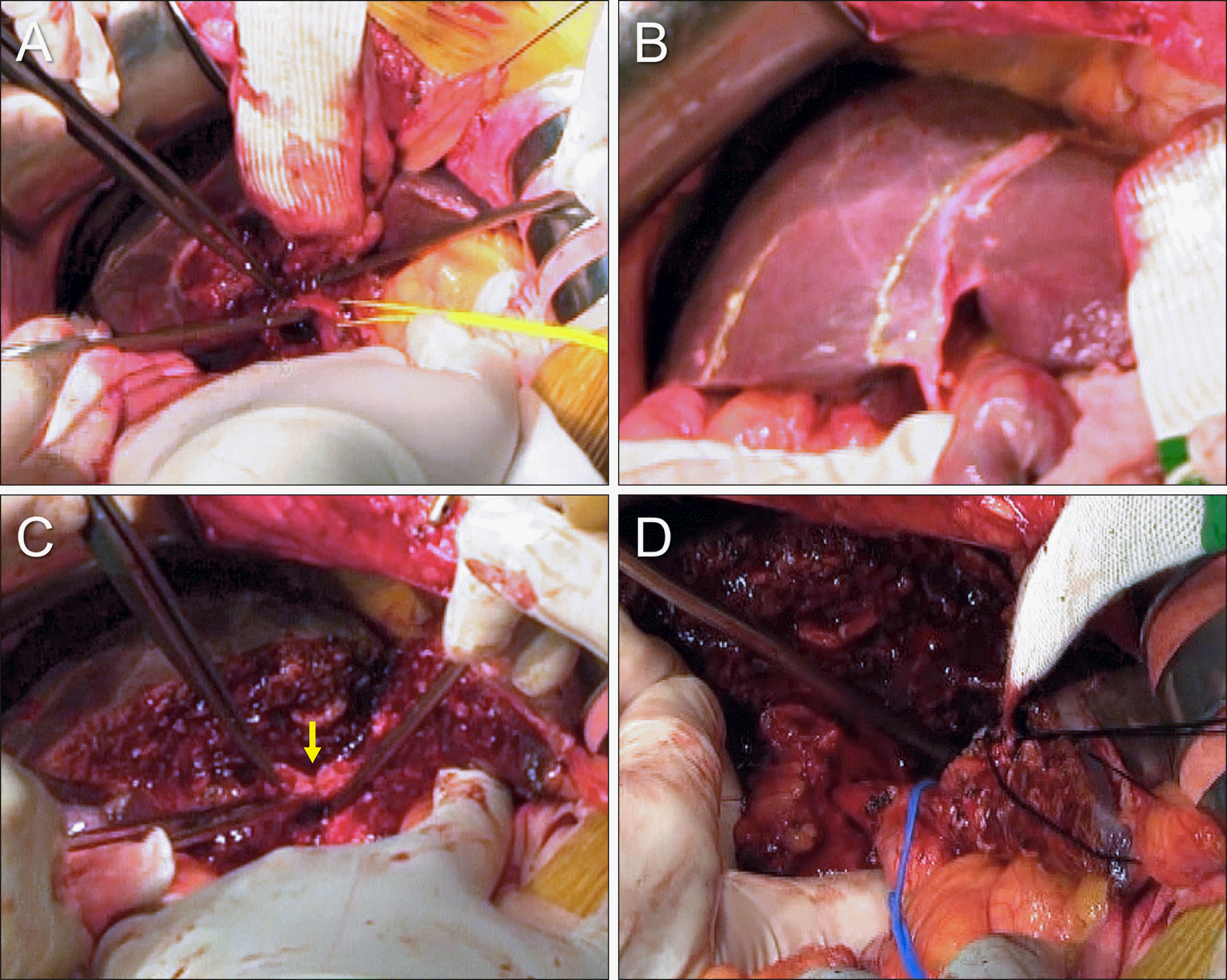
Fig. 10
Intraoperative photographs showing the surgical procedure for resection of segments I+IV and bile duct. (A) The right-sided hepatic parenchymal transection is performed along the hemi-liver discoloration line. (B) The right anterior and posterior section ducts are transected (arrow). (C) The segment IV parenchyma is separated from the right and left remnant livers. (D) The resected specimen includes the segments I+IV, gallbladder and extrahepatic bile duct.
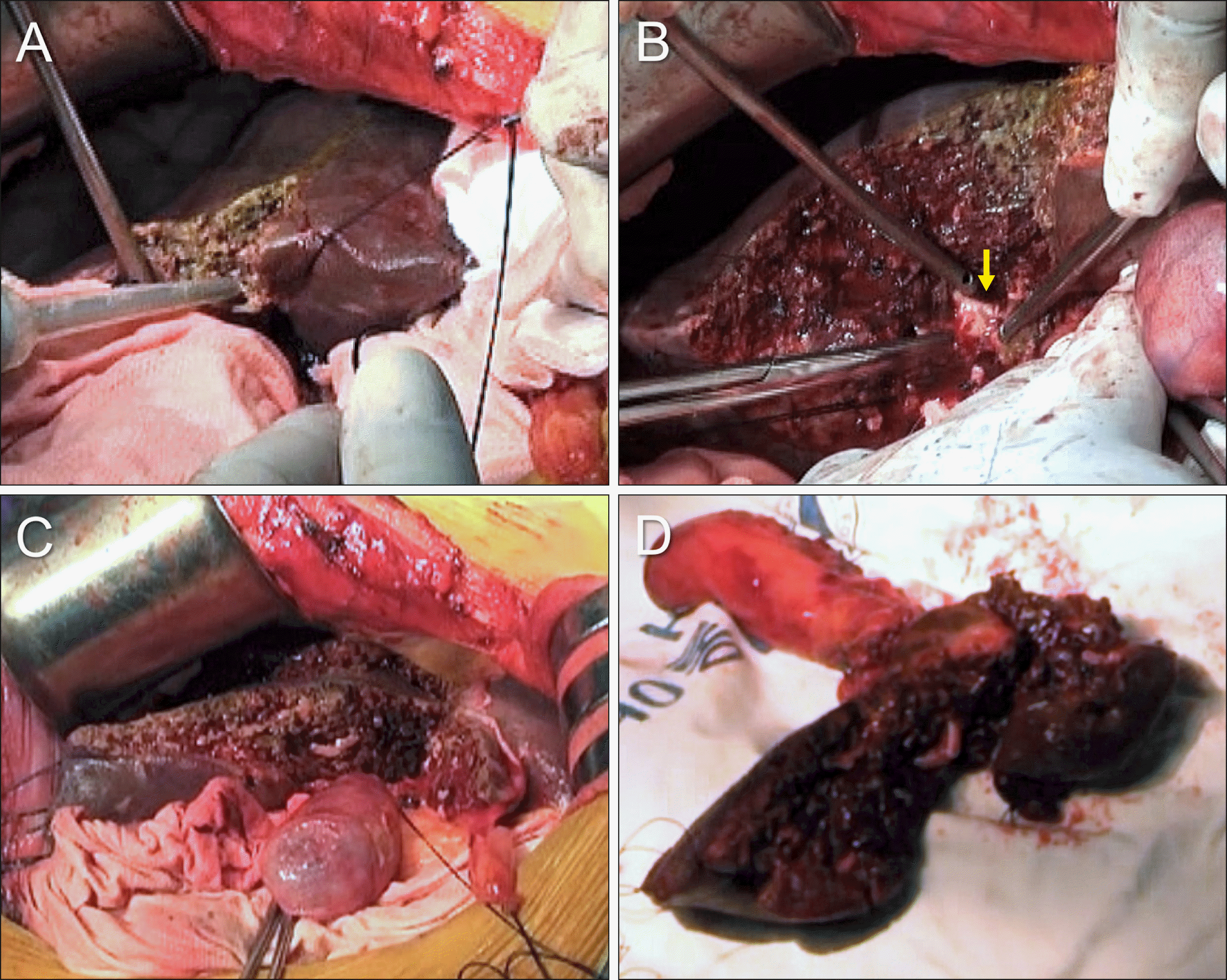
Fig. 11
Intraoperative photographs showing the surgical procedure for segments I+IV resection and bile duct resection. (A) The opening of the right anterior section duct is conjoined with the opening of the right posterior duct via unification ductoplasty (arrow). (B) Openings of the 4 right hepatic ducts and 3 left hepatic ducts are exposed. Silastic tubes are inserted to identify the duct openings. (C) The 4-cm- wide conjoined right hepatic duct opening is reconstructed by single hepaticojejunostomy. (D) The 2.5-cm-wide left hepatic duct opening is reconstructed by single hepaticojejunostomy.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download