1. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017; 9(396):eaal3653. PMID:
28659436.

2. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30(3):269–271. PMID:
32020029.



3. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. PMID:
32423584.


4. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020; 383(19):1813–1826. PMID:
32445440.


5. Kalligeros M, Tashima KT, Mylona EK, Rybak N, Flanigan TP, Farmakiotis D, et al. Remdesivir use compared with supportive care in hospitalized patients with severe COVID-19: a single-center experience. Open Forum Infect Dis. 2020; 7(10):ofaa319. PMID:
33117850.

6. Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. Forthcoming. 2020.

7. WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for COVID-19 - interim WHO solidarity trial results. N Engl J Med. 2021; 384(6):497–511. PMID:
33264556.


8. Lee C, Ahn MY, Byeon K, Choi JP, Hahm C, Kim H, et al. Clinical experience with use of remdesivir in the treatment of severe acute respiratory syndrome coronavirus 2: a case series. Infect Chemother. 2020; 52(3):369–380. PMID:
32757500.

9. Yoo JH. Uncertainty about the efficacy of remdesivir on COVID-19. J Korean Med Sci. 2020; 35(23):e221. PMID:
32537956.

10. Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci. 2020; 35(30):e280. PMID:
32743995.

12. Huh HJ, Hong KH, Kim TS, Song SH, Roh KH, Lee H, et al. Surveillance of coronavirus disease 2019 (COVID-19) testing in clinical laboratories in Korea. Ann Lab Med. 2021; 41(2):225–229. PMID:
33063685.



13. Ko JH, Lim JU, Choi JY, Oh HS, Yoo H, Jhun BW, et al. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: a retrospective cohort study. Clin Microbiol Infect. 2020; 26(5):646.e9–646.e14.

14. Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. 2013; 68(8):1872–1880. PMID:
23572228.



15. Boikos C, Caya C, Doll MK, Kraicer-Melamed H, Dolph M, Delisle G, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017; 72(6):1556–1573. PMID:
28204554.


16. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021; 384(3):238–251. PMID:
33332778.


17. Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021; 384(3):229–237. PMID:
33113295.


18. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020; 585(7824):273–276. PMID:
32516797.



19. Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, Choi WS, et al. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. 2020; 52(2):281–304. PMID:
32342676.



20. Kim JW, Kim EJ, Kwon HH, Jung CY, Kim KC, Choe JY, et al. Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019. Korean J Intern Med. 2021; 36(Suppl 1):S253–S263. PMID:
32536150.

21. RECOVERY Collaborative Group. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020; 383(21):2030–2040. PMID:
33031652.

22. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020; 382(25):2411–2418. PMID:
32379955.


23. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020; 382(19):1787–1799. PMID:
32187464.

24. Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021; 384(7):610–618. PMID:
33406353.


25. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med. Forthcoming. 2021.
26. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021; 384(7):619–629. PMID:
33232588.


27. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020; 395(10238):1695–1704. PMID:
32401715.


28. Hoang T, Anh TT. Treatment options for severe acute respiratory syndrome, middle east respiratory syndrome, and coronavirus disease 2019: a review of clinical evidence. Infect Chemother. 2020; 52(3):317–334. PMID:
32869558.



29. Im JH, Nahm CH, Baek JH, Kwon HY, Lee JS. Convalescent plasma therapy in coronavirus disease 2019: a case report and suggestions to overcome obstacles. J Korean Med Sci. 2020; 35(26):e239. PMID:
32627442.

30. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020; 35(14):e149. PMID:
32281317.

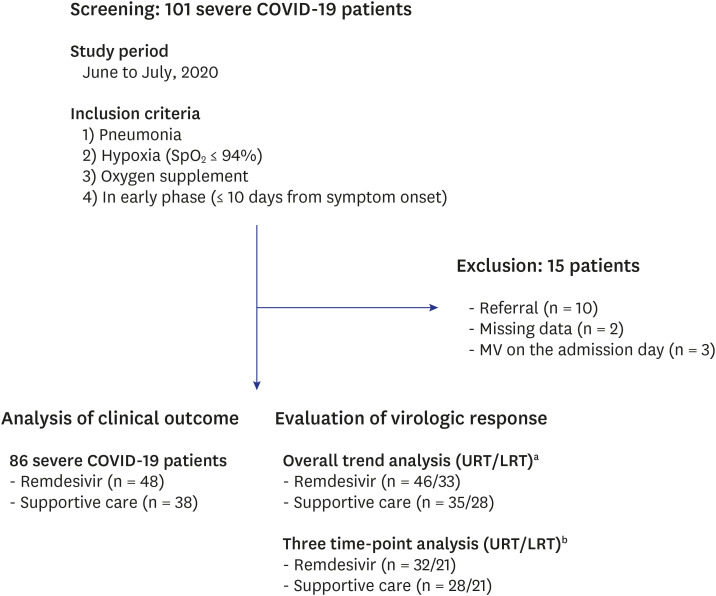
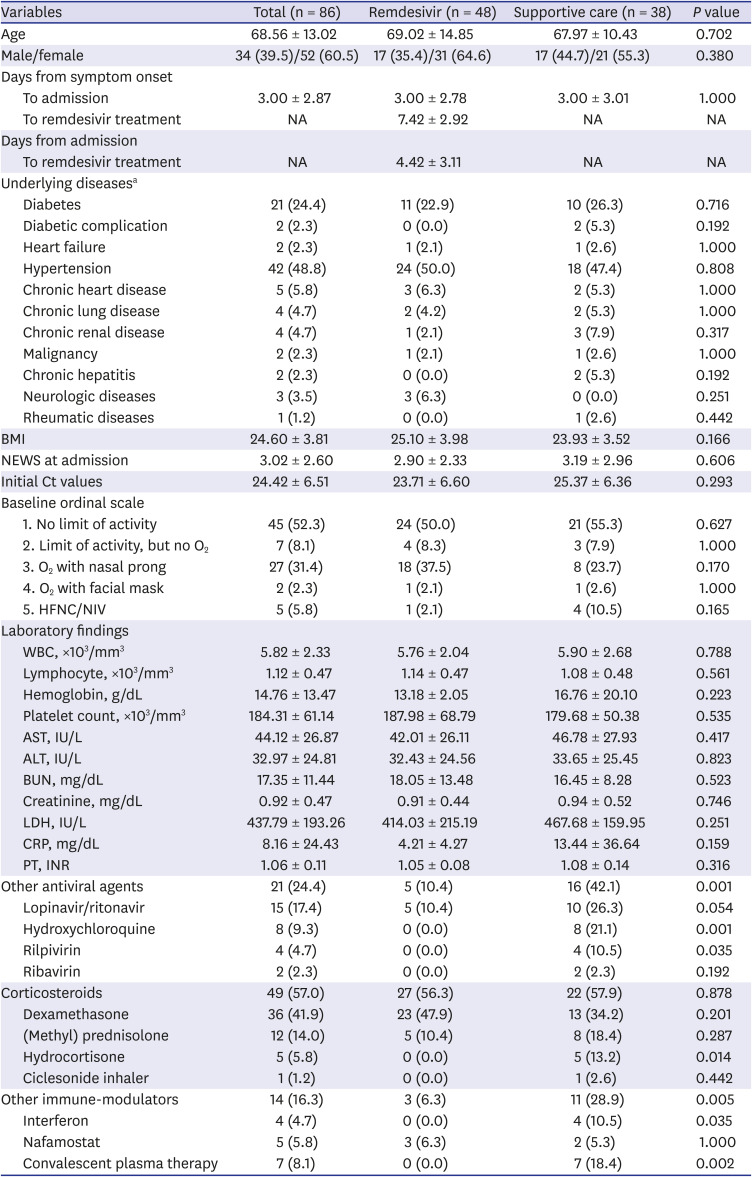
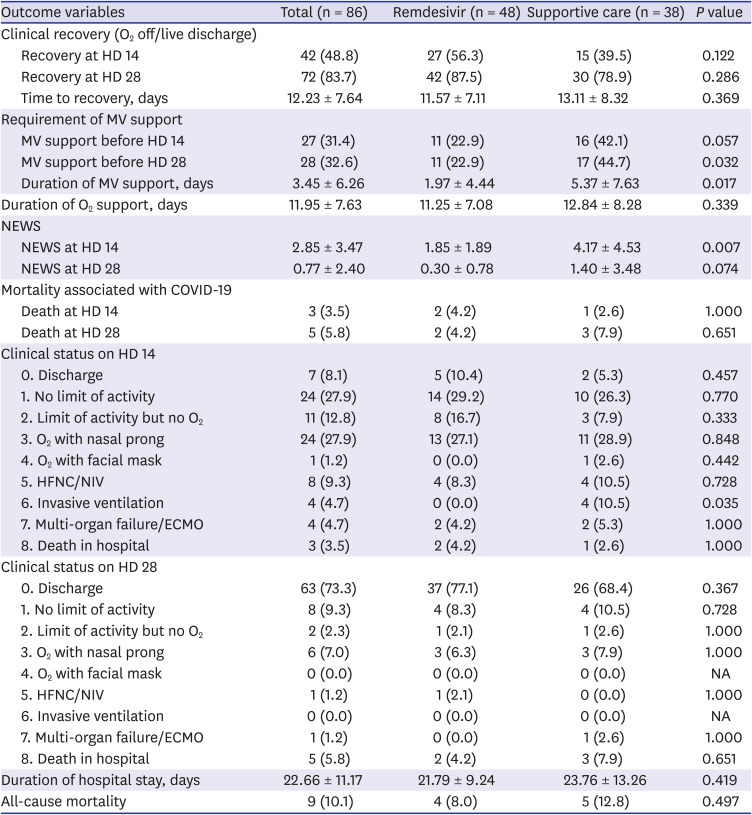
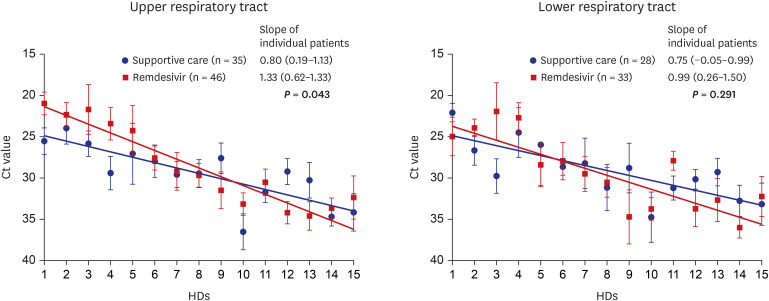




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download