1. Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009; 14(6):358–366. PMID:
19783238.


2. Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health. 2013; 18(2):86–90. PMID:
24421662.



3. Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004; 144(6):799–803. PMID:
15192629.


4. Dumpa V, Bhandari V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. 2018; 42(7):444–452. PMID:
30343941.


5. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003; 8(1):63–71. PMID:
12667831.


6. Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011; 183(12):1715–1722. PMID:
21471086.



7. The Executive Committee of Korean Neonatal Network. 2016 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2017.
8. Jo HS, Cho KH, Cho SI, Song ES, Kim BI. Recent changes in the incidence of bronchopulmonary dysplasia among very low birth weight infants in Korea. J Korean Med Sci. 2015; 30(Suppl 1):S81–S87. PMID:
26566362.
9. Chang YS, Ahn SY, Park WS. The establishment of the Korean Neonatal Network (KNN). Neonatal Med. 2013; 20(2):169–178.

10. The Executive Committee of Korean Neonatal Network. 2013 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2014.
11. The Executive Committee of Korean Neonatal Network. 2014 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2015.
12. The Executive Committee of Korean Neonatal Network. 2015 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2016.
13. The Executive Committee of Korean Neonatal Network. 2016 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2017.
14. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005; 116(6):1353–1360. PMID:
16322158.


15. The Executive Committee of Korean Neonatal Network. 2017 Korean Neonatal Network Annual Report. Cheongju: Korea Centers for Disease Control and Prevention;2018.
16. Onland W, Debray TP, Laughon MM, Miedema M, Cools F, Askie LM, et al. Clinical prediction models for bronchopulmonary dysplasia: a systematic review and external validation study. BMC Pediatr. 2013; 13(1):207–226. PMID:
24345305.



17. Parker RA, Lindstrom DP, Cotton RB. Improved survival accounts for most, but not all, of the increase in bronchopulmonary dysplasia. Pediatrics. 1992; 90(5):663–668. PMID:
1408535.

18. Henderson-Smart DJ, Hutchinson JL, Donoghue DA, Evans NJ, Simpson JM, Wright I, et al. Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2006; 91(1):F40–5. PMID:
16131530.

19. Chien LY, Whyte R, Thiessen P, Walker R, Brabyn D, Lee SK, et al. Snap-II predicts severe intraventricular hemorrhage and chronic lung disease in the neonatal intensive care unit. J Perinatol. 2002; 22(1):26–30. PMID:
11840239.


20. Rivera L, Siddaiah R, Oji-Mmuo C, Silveyra GR, Silveyra P. Biomarkers for bronchopulmonary dysplasia in the preterm infant. Front Pediatr. 2016; 4:33. PMID:
27065351.



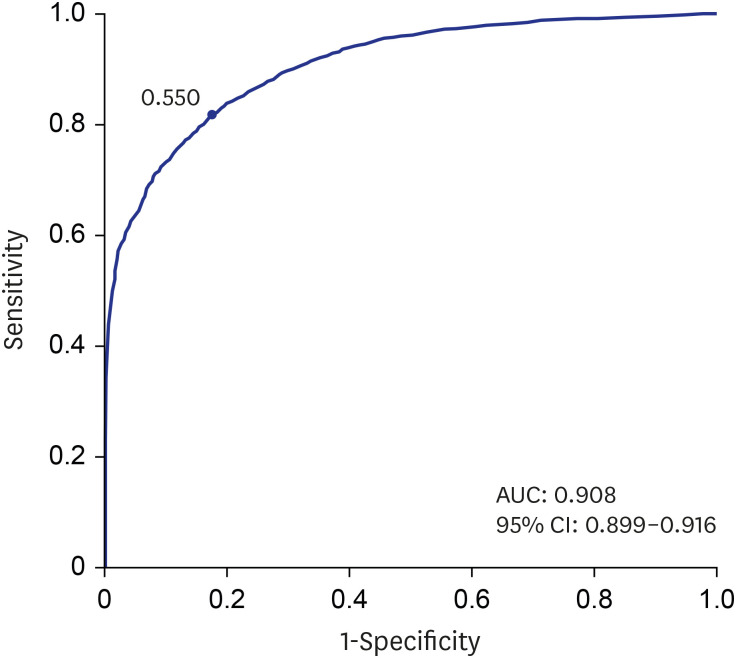
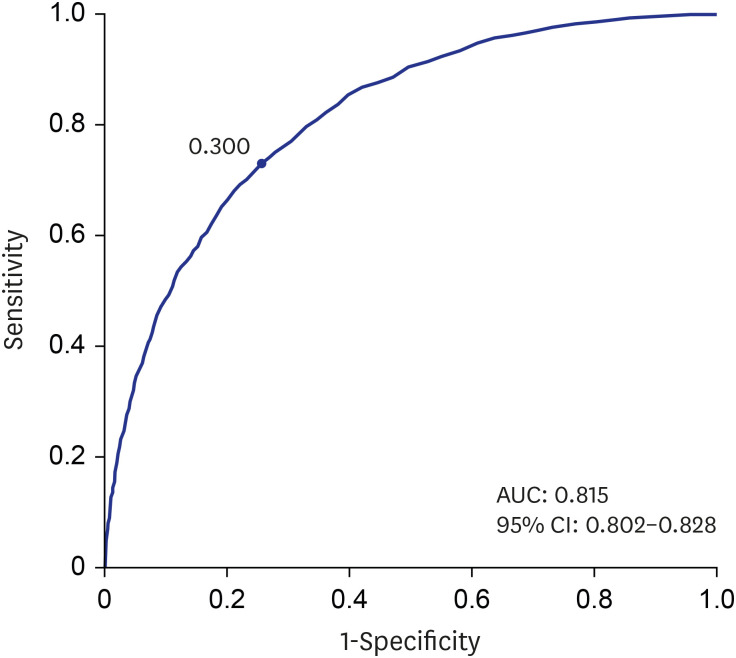
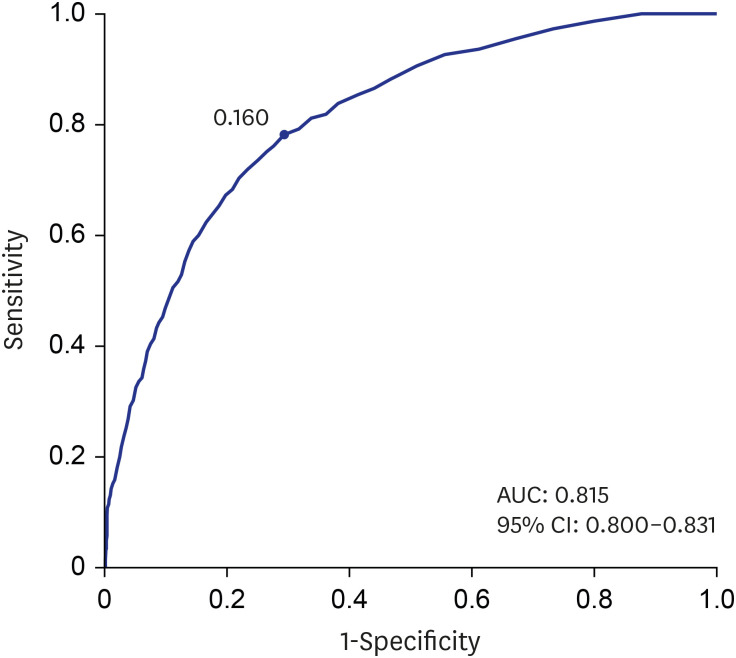
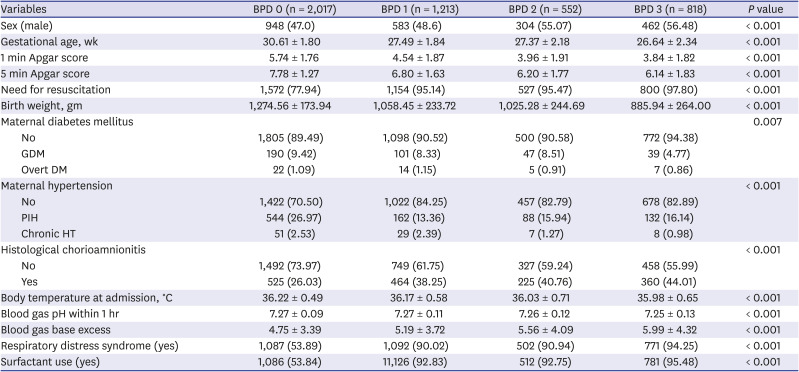




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download