1. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002; 46(9):2287–2293. PMID:
12355475.


2. Shim TS. Diagnosis and treatment of latent tuberculosis infection due to initiation of anti-tnf therapy. Tuberc Respir Dis (Seoul). 2014; 76(6):261–268. PMID:
25024719.



3. Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020; 35(2):269–275. PMID:
32131570.



4. Iannone F, Cantini F, Lapadula G. Diagnosis of latent tuberculosis and prevention of reactivation in rheumatic patients receiving biologic therapy: international recommendations. J Rheumatol Suppl. 2014; 91(0):41–46. PMID:
24788999.


5. Ponce de León D, Acevedo-Vásquez E, Sánchez-Torres A, Cucho M, Alfaro J, Perich R, et al. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005; 64(9):1360–1361. PMID:
16100342.


6. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008; 149(3):177–184. PMID:
18593687.



7. Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis. 2006; 6(1):47. PMID:
16539718.



8. World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Geneva: World Health Organization;2018.
9. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 3rd ed. Cheongju: Korea Centers for Disease Control and Prevention;2017.
10. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016; 68(1):1–25. PMID:
26545825.


11. Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016; 75(6):1133–1138. PMID:
26318385.


12. Maiga M, Lun S, Guo H, Winglee K, Ammerman NC, Bishai WR. Risk of tuberculosis reactivation with tofacitinib (CP-690550). J Infect Dis. 2012; 205(11):1705–1708. PMID:
22474037.



13. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 1st ed. Seoul: Joint Committee for the Revision of Korean Guidelines for Tuberculosis;2011.
14. Takahashi H, Shigehara K, Yamamoto M, Suzuki C, Naishiro Y, Tamura Y, et al. Interferon gamma assay for detecting latent tuberculosis infection in rheumatoid arthritis patients during infliximab administration. Rheumatol Int. 2007; 27(12):1143–1148. PMID:
17503048.

15. Lee KH, Jung SY, Ha YJ, Lee SK, Park YB. Tuberculin reaction is not attenuated in patients with rheumatoid arthritis living in a region with intermediate burden of tuberculosis. Rheumatol Int. 2012; 32(5):1421–1424. PMID:
21442172.


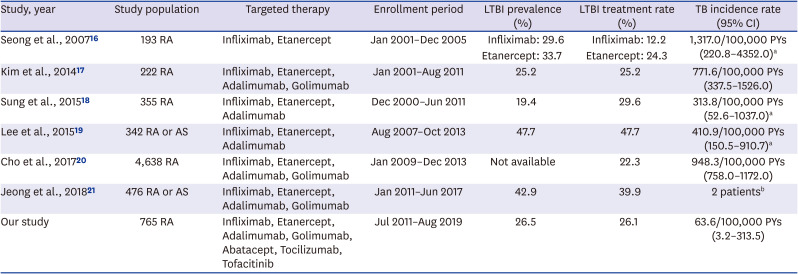
16. Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007; 34(4):706–711. PMID:
17309133.

17. Kim HW, Park JK, Yang JA, Yoon YI, Lee EY, Song YW, et al. Comparison of tuberculosis incidence in ankylosing spondylitis and rheumatoid arthritis during tumor necrosis factor inhibitor treatment in an intermediate burden area. Clin Rheumatol. 2014; 33(9):1307–1312. PMID:
24057090.


18. Sung YK, Cho SK, Won S, Choi CB, Kim TH, Jun JB, et al. Incidence of tuberculosis in rheumatoid arthritis patients using anti-tumor necrosis factor agents following latent tuberculosis infection screening strategies. J Rheum Dis. 2015; 22(4):223–230.

19. Lee H, Park HY, Jeon K, Jeong BH, Hwang JW, Lee J, et al. QuantiFERON-TB Gold In-Tube assay for screening arthritis patients for latent tuberculosis infection before starting anti-tumor necrosis factor treatment. PLoS One. 2015; 10(3):e0119260. PMID:
25746854.

20. Cho SK, Kim D, Won S, Han M, Lee J, Jang EJ, et al. Safety of resuming biologic DMARDs in patients who develop tuberculosis after anti-TNF treatment. Semin Arthritis Rheum. 2017; 47(1):102–107. PMID:
28216194.


21. Jeong DH, Kang J, Jung YJ, Yoo B, Lee CK, Kim YG, et al. Comparison of latent tuberculosis infection screening strategies before tumor necrosis factor inhibitor treatment in inflammatory arthritis: IGRA-alone versus combination of TST and IGRA. PLoS One. 2018; 13(7):e0198756. PMID:
29975703.

22. Harris J, Keane J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol. 2010; 161(1):1–9. PMID:
20491796.



23. Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol. 2011; 186(7):4234–4243. PMID:
21383241.



24. Kim JH, Cho SK, Han M, Choi CB, Kim TH, Jun JB, et al. Factors influencing discrepancies between the QuantiFERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum. 2013; 42(4):424–432. PMID:
22858451.


25. Pyo J, Cho SK, Kim D, Sung YK. Systemic review: agreement between the latent tuberculosis screening tests among patients with rheumatic diseases. Korean J Intern Med. 2018; 33(6):1241–1251. PMID:
29277097.

26. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293(22):2756–2761. PMID:
15941805.

27. Lee JH, Sohn HS, Chun JH, Kim HA, Suh CH, Lee YW, et al. Poor agreement between QuantiFERON-TB Gold test and tuberculin skin test results for the diagnosis of latent tuberculosis infection in rheumatoid arthritis patients and healthy controls. Korean J Intern Med. 2014; 29(1):76–84. PMID:
24574836.



28. Son CN, Jun JB, Kim JH, Sung IH, Yoo DH, Kim TH. Follow-up testing of interferon-gamma release assays are useful in ankylosing spondylitis patients receiving anti-tumor necrosis factor alpha for latent tuberculosis infection. J Korean Med Sci. 2014; 29(8):1090–1093. PMID:
25120318.



29. Kim HW, Kim JS. Treatment of latent tuberculosis infection and its clinical efficacy. Tuberc Respir Dis (Seoul). 2018; 81(1):6–12. PMID:
29332319.


30. Yun JW, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Diagnosis and treatment of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. J Korean Med Sci. 2007; 22(5):779–783. PMID:
17982222.



31. Sung YK, Cho SK, Kim D, Won S, Choi CB, Kim TH, et al. Isoniazid treatment for latent tuberculosis infection is tolerable for rheumatoid arthritis patients receiving tumor necrosis factor inhibitor therapy. Korean J Intern Med. 2018; 33(5):1016–1024. PMID:
28288508.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download