1. Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992; (277):12–21.
2. Seamon J, Keller T, Saleh J, Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis (Egypt). 2012; 2012:601763.

3. Yoon BH, Jones LC, Chen CH, Cheng EY, Cui Q, Drescher W, et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 1: glucocorticoid-associated osteonecrosis. J Arthroplasty. 2019; 34(1):163–168.e1. PMID:
30348552.


4. Yoon BH, Jones LC, Chen CH, Cheng EY, Cui Q, Drescher W, et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 2: alcohol-associated osteonecrosis. J Arthroplasty. 2019; 34(1):169–174.e1. PMID:
30348559.


5. Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015; 97(19):1604–1627. PMID:
26446969.

6. Ikeuchi K, Hasegawa Y, Seki T, Takegami Y, Amano T, Ishiguro N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol. 2015; 25(2):278–281. PMID:
25036228.


7. Kang JS, Park S, Song JH, Jung YY, Cho MR, Rhyu KH. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplasty. 2009; 24(8):1178–1183. PMID:
19640674.

8. Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J (Engl). 2015; 128(21):2843–2850. PMID:
26521779.


9. Mont MA, Salem HS, Piuzzi NS, Goodman SB, Jones LC. Nontraumatic osteonecrosis of the femoral head: where do we stand today?: a 5-year update. J Bone Joint Surg Am. 2020; 102(12):1084–1099. PMID:
32282421.

10. Arlet JD, Fauchier C, Thiechart M. Histopathology of nontraumatic necrosis of the femoral head: topographic and evolutive aspects. In : Arlet J, Ficat RP, Hungerford DS, editors. Bone Circulation. Baltimore, MD: Williams & Wilkins;1984. p. 296–305.
11. Hauzeur JP, Perlmutter N, Appelboom T, Pasteels JL. Medullary impairment at early stage of non-traumatic osteonecrosis of the femoral head. Rheumatol Int. 1991; 11(4-5):215–217. PMID:
1784891.


12. Hofmann S, Engel A, Neuhold A, Leder K, Kramer J, Plenk H Jr. Bone-marrow oedema syndrome and transient osteoporosis of the hip. An MRI-controlled study of treatment by core decompression. J Bone Joint Surg Br. 1993; 75(2):210–216. PMID:
8444939.


13. Hauzeur JP, Sintzoff S Jr, Appelboom T, De Maertelaer V, Bentin J, Pasteels JL. Relationship between magnetic resonance imaging and histologic findings by bone biopsy in nontraumatic osteonecrosis of the femoral head. J Rheumatol. 1992; 19(3):385–392. PMID:
1578452.

14. Hauzeur JP, Pasteels JL, Orloff S. Bilateral non-traumatic aseptic osteonecrosis in the femoral head. An experimental study of incidence. J Bone Joint Surg Am. 1987; 69(8):1221–1225. PMID:
2822722.


15. Koo KH, Kim R, Cho SH, Song HR, Lee G, Ko GH. Angiography, scintigraphy, intraosseous pressure, and histologic findings in high-risk osteonecrotic femoral heads with negative magnetic resonance images. Clin Orthop Relat Res. 1994; (308):127–138.

16. Koo KH, Jeong ST, Jones JP Jr. Borderline necrosis of the femoral head. Clin Orthop Relat Res. 1999; (358):158–165.

17. Sugano N, Nishii T, Shibuya T, Nakata K, Masuhara K, Takaoka K. Contralateral hip in patients with unilateral nontraumatic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997; 334:85–90.

18. Goetz JE, Robinson DA, Pedersen DR, Conzemius MG, Brown TD. Cryoinsult parameter effects on the histologically apparent volume of experimentally induced osteonecrotic lesions. J Orthop Res. 2011; 29(6):931–937. PMID:
21259339.



19. Yoon BH, Mont MA, Koo KH, Chen CH, Cheng EY, Cui Q, et al. The 2019 revised version of association research circulation osseous staging system of osteonecrosis of the femoral head. J Arthroplasty. 2020; 35(4):933–940. PMID:
31866252.


20. Jones JP Jr. Concepts of etiology and early pathogenesis of osteonecrosis. Instr Course Lect. 1994; 43:499–512. PMID:
9097180.

21. Jones JP Jr, Peltier LF. Alcoholism, hypercortisonism, fat embolism and osseous avascular necrosis. 1971. Clin Orthop Relat Res. 2001; 393:4–12.
22. Asano T, Takahashi KA, Fujioka M, Inoue S, Okamoto M, Sugioka N, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2003; 13(11):675–682. PMID:
14583680.


23. Chao YC, Wang SJ, Chu HC, Chang WK, Hsieh TY. Investigation of alcohol metabolizing enzyme genes in Chinese alcoholics with avascular necrosis of hip joint, pancreatitis and cirrhosis of the liver. Alcohol Alcohol. 2003; 38(5):431–436. PMID:
12915519.


24. Koo KH, Lee JS, Lee YJ, Kim KJ, Yoo JJ, Kim HJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res. 2006; 24(8):1722–1728. PMID:
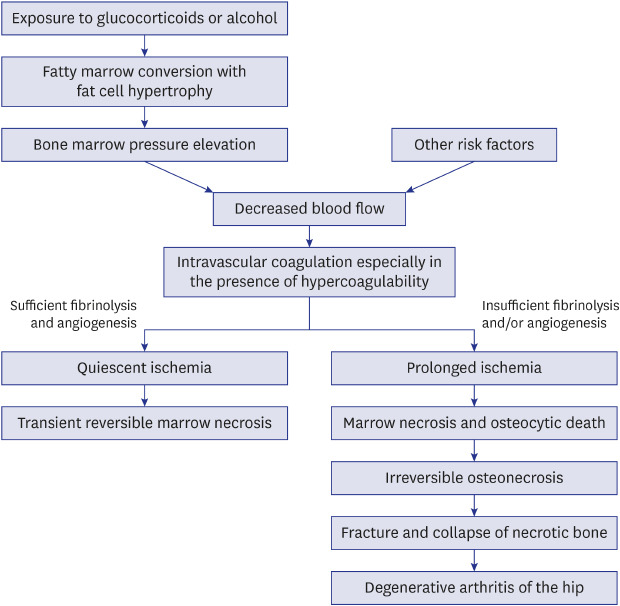
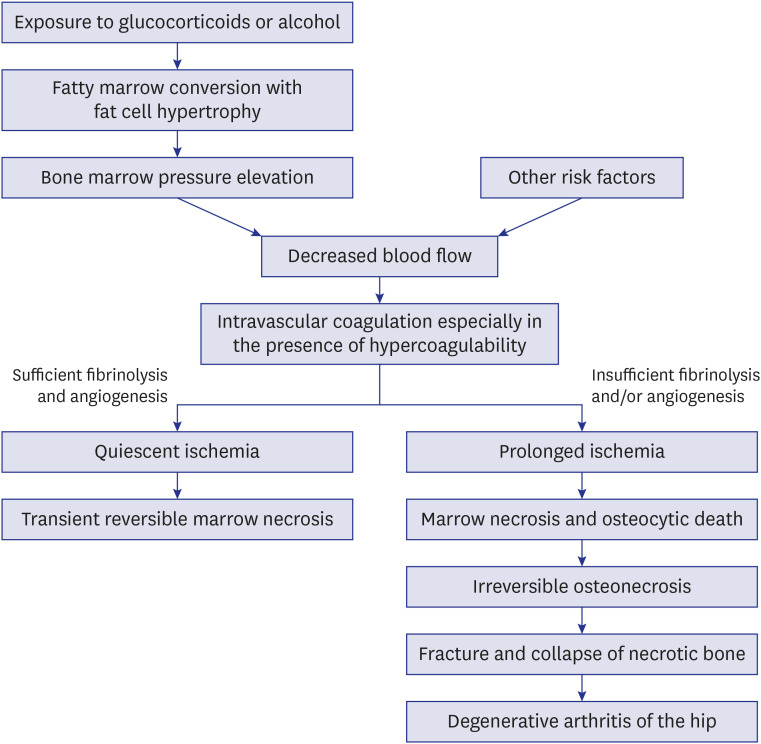
16779830.


25. Chang JD, Hur M, Lee SS, Yoo JH, Lee KM. Genetic background of nontraumatic osteonecrosis of the femoral head in the Korean population. Clin Orthop Relat Res. 2008; 466(5):1041–1046. PMID:
18350352.

26. Kim TH, Hong JM, Lee J, Oh B, Park EK, Lee CK, et al. Promoter polymorphisms of the vascular endothelial growth factor gene is associated with an osteonecrosis of the femoral head in the Korean population. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society. 2008; 16(3):287–291.
27. Kim TH, Hong JM, Oh B, Cho YS, Lee JY, Kim HL, et al. Genetic association study of polymorphisms in the catalase gene with the risk of osteonecrosis of the femoral head in the Korean population. Osteoarthritis Cartilage. 2008; 16(9):1060–1066. PMID:
18353692.


28. Lee YJ, Lee JS, Kang EH, Lee YK, Kim SY, Song YW, et al. Vascular endothelial growth factor polymorphisms in patients with steroid-induced femoral head osteonecrosis. J Orthop Res. 2012; 30(1):21–27. PMID:
21710604.


29. Glueck CJ, Freiberg RA, Boppana S, Wang P. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am. 2008; 90(10):2220–2229. PMID:
18829920.


30. Chandler FA. Coronary disease of the hip. J Int Coll Surg. 1948; 11(1):34–36. PMID:
18910401.

31. Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011; 60(11):1500–1510. PMID:
21864867.


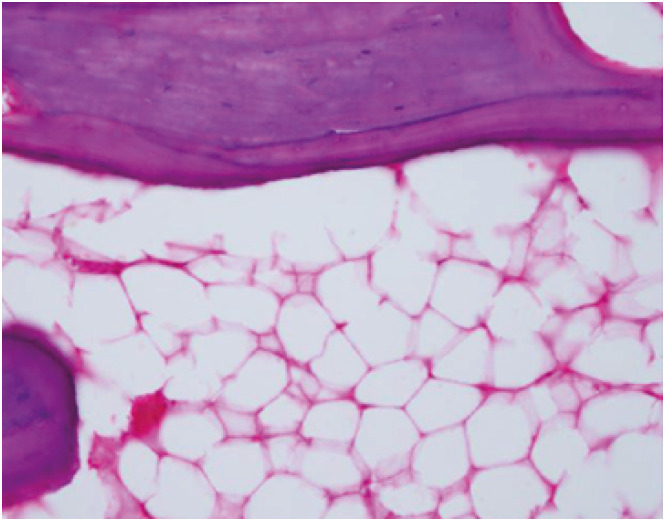
32. Motomura G, Yamamoto T, Miyanishi K, Yamashita A, Sueishi K, Iwamoto Y. Bone marrow fat-cell enlargement in early steroid-induced osteonecrosis--a histomorphometric study of autopsy cases. Pathol Res Pract. 2005; 200(11-12):807–811. PMID:
15792124.


33. Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002; 30(1):185–190. PMID:
11792583.


34. Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977; 59(6):729–735. PMID:
908695.


35. Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997; 79(7):1054–1063. PMID:
9234882.


36. Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006; 88(Suppl 3):148–154.

37. Zhou Z, Hua Y, Liu J, Zuo D, Wang H, Chen Q, et al. Association of ABCB1/MDR1 polymorphisms in patients with glucocorticoid-induced osteonecrosis of the femoral head: evidence for a meta-analysis. Gene. 2015; 569(1):34–40. PMID:
25791493.


38. Hao Y, Guo H, Xu Z, Qi H, Wang Y, Lu C, et al. The relationship between apolipoprotein genes polymorphisms and susceptibility to osteonecrosis of the femoral head: a meta-analysis. Lipids Health Dis. 2018; 17(1):192. PMID:
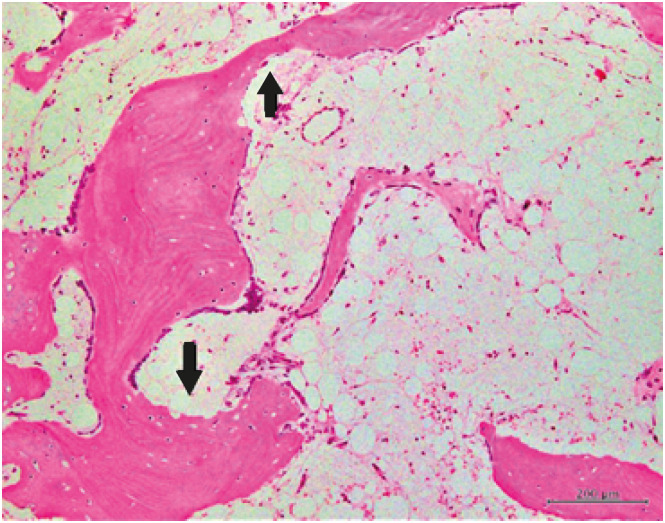
30119683.



39. Koo KH, Dussault R, Kaplan P, Kim R, Ahn IO, Christopher J, et al. Age-related marrow conversion in the proximal metaphysis of the femur: evaluation with T1-weighted MR imaging. Radiology. 1998; 206(3):745–748. PMID:
9494495.


40. Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: implications for treatment. Orthop Clin North Am. 1985; 16(4):635–654. PMID:
3903603.


41. Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985; 14(1):10–19. PMID:
3895447.


42. Lausten GS, Arnoldi CC. Blood perfusion uneven in femoral head osteonecrosis. Doppler flowmetry and intraosseous pressure in 12 cases. Acta Orthop Scand. 1993; 64(5):533–536. PMID:
8237319.


43. Humphreys S, Spencer JD, Tighe JR Jr, Cumming RR. The femoral head in osteonecrosis. A quantitative study of osteocyte population. J Bone Joint Surg Br. 1989; 71(2):205–208. PMID:
2647752.


44. James J, Steijn-Myagkaya GL. Death of osteocytes. Electron microscopy after in vitro ischaemia. J Bone Joint Surg Br. 1986; 68(4):620–624. PMID:
3733842.


45. Nadel SN, Debatin JF, Richardson WJ, Hedlund LW, Senft C, Rizk WS, et al. Detection of acute avascular necrosis of the femoral head in dogs: dynamic contrast-enhanced MR imaging vs spin-echo and STIR sequences. AJR Am J Roentgenol. 1992; 159(6):1255–1261. PMID:
1442396.


46. Fondi C, Franchi A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab. 2007; 4(1):21–26. PMID:
22460748.


47. Jones LC, Mont MA, Le TB, Petri M, Hungerford DS, Wang P, et al. Procoagulants and osteonecrosis. J Rheumatol. 2003; 30(4):783–791. PMID:
12672200.

48. Pierre-Jacques H, Glueck CJ, Mont MA, Hungerford DS. Familial heterozygous protein-S deficiency in a patient who had multifocal osteonecrosis. A case report. J Bone Joint Surg Am. 1997; 79(7):1079–1084. PMID:
9234887.


49. Glueck CJ, Freiberg R, Tracy T, Stroop D, Wang P. Thrombophilia and hypofibrinolysis: pathophysiologies of osteonecrosis. Clin Orthop Relat Res. 1997; (334):43–56. PMID:
9005895.

50. Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Genetic background of osteonecrosis: associated with thrombophilic mutations? Clin Orthop Relat Res. 2004; (422):251–255.
51. Björkman A, Svensson PJ, Hillarp A, Burtscher IM, Rünow A, Benoni G. Factor V Leiden and prothrombin gene mutation: risk factors for osteonecrosis of the femoral head in adults. Clin Orthop Relat Res. 2004; (425):168–172.
52. Glueck CJ, Fontaine RN, Gruppo R, Stroop D, Sieve-Smith L, Tracy T, et al. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res. 1999; (366):133–146. PMID:
10627727.

53. Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001; (386):19–33. PMID:
11347834.

54. Seleznick MJ, Silveira LH, Espinoza LR. Avascular necrosis associated with anticardiolipin antibodies. J Rheumatol. 1991; 18(9):1416–1417. PMID:
1757948.

55. Korompilias AV, Gilkeson GS, Ortel TL, Seaber AV, Urbaniak JR. Anticardiolipin antibodies and osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997; (345):174–180.

56. Vakil N, Sparberg M. Steroid-related osteonecrosis in inflammatory bowel disease. Gastroenterology. 1989; 96(1):62–67. PMID:
2909438.


57. Hauzeur JP, Malaise M, Gangji V. Osteonecrosis in inflammatory bowel diseases: a review of the literature. Acta Gastroenterol Belg. 2009; 72(3):327–334. PMID:
19902866.

58. Golding JS. Conditions of the hip associated with hemoglobinopathies. Clin Orthop Relat Res. 1973; (90):22–28. PMID:
4689124.
59. Hernigou P, Galacteros F, Bachir D, Goutallier D. Deformities of the hip in adults who have sickle-cell disease and had avascular necrosis in childhood. A natural history of fifty-two patients. J Bone Joint Surg Am. 1991; 73(1):81–92. PMID:
1985998.


60. Sakamoto Y, Yamamoto T, Sugano N, Takahashi D, Watanabe T, Atsumi T, et al. Genome-wide association study of idiopathic osteonecrosis of the femoral head. Sci Rep. 2017; 7(1):15035. PMID:
29118346.

61. Kabata T, Matsumoto T, Yagishita S, Wakayama T, Iseki S, Tomita K. Vascular endothelial growth factor in rabbits during development of corticosteroid-induced osteonecrosis: a controlled experiment. J Rheumatol. 2008; 35(12):2383–2390. PMID:
19004033.


62. Mitchell DG, Kressel HY, Arger PH, Dalinka M, Spritzer CE, Steinberg ME. Avascular necrosis of the femoral head: morphologic assessment by MR imaging, with CT correlation. Radiology. 1986; 161(3):739–742. PMID:
3786725.


63. Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960; 42-A:138–143. PMID:
13849861.


64. Catto M. A histological study of avascular necrosis of the femoral head after transcervical fracture. J Bone Joint Surg Br. 1965; 47(4):749–776. PMID:
5846780.


65. McCarthy EF. Aseptic necrosis of bone. An historic perspective. Clin Orthop Relat Res. 1982; (168):216–221. PMID:
7049484.
66. Koo KH, Ahn IO, Kim R, Song HR, Jeong ST, Na JB, et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999; 213(3):715–722. PMID:
10580944.


67. Atsumi T, Kuroki Y, Yamano K. A microangiographic study of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989; (246):186–194. PMID:
2766607.

68. Chen J, Liu W, Cao Y, Zhang X, Guo Y, Zhu Y, et al. MMP-3 and MMP-8 single-nucleotide polymorphisms are related to alcohol-induced osteonecrosis of the femoral head in Chinese males. Oncotarget. 2017; 8(15):25177–25188. PMID:
28445942.



69. Du J, Liu W, Jin T, Zhao Z, Bai R, Xue H, et al. A single-nucleotide polymorphism in MMP9 is associated with decreased risk of steroid-induced osteonecrosis of the femoral head. Oncotarget. 2016; 7(42):68434–68441. PMID:
27637086.
70. Chen B, Du Z, Dong X, Li Z, Wang Q, Chen G, et al. Association of variant interactions in
RANK,
RANKL,
OPG,
TRAF6, and
NFATC1 genes with the development of osteonecrosis of the femoral head. DNA Cell Biol. 2019; 38(7):734–746. PMID:
31149839.

71. Wang D, Haile A, Jones LC. Dexamethasone-induced lipolysis increases the adverse effect of adipocytes on osteoblasts using cells derived from human mesenchymal stem cells. Bone. 2013; 53(2):520–530. PMID:
23328495.


72. Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: an abridged communication. Clin Orthop Relat Res. 1978; (130):47–50.
73. Glimcher MJ, Kenzora JE. Nicolas Andry award. The biology of osteonecrosis of the human femoral head and its clinical implications: 1. Tissue biology. Clin Orthop Relat Res. 1979; (138):284–309.
74. Mitchell DG, Rao VM, Dalinka MK, Spritzer CE, Alavi A, Steinberg ME, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987; 162(3):709–715. PMID:
3809484.


75. Curtiss PH Jr, Kincaid WE. Transitory demineralization of the hip in pregnancy. A report of three cases. J Bone Joint Surg Am. 1959; 41-A:1327–1333. PMID:
13849487.
76. Hayes CW, Conway WF, Daniel WW. MR imaging of bone marrow edema pattern: transient osteoporosis, transient bone marrow edema syndrome, or osteonecrosis. Radiographics. 1993; 13(5):1001–1011. PMID:
8210586.


77. Kim SY, Koo KH, Suh KT, Kim YS, Cho YJ, Min BW, et al. Fatty marrow conversion of the proximal femoral metaphysis in transient bone marrow edema syndrome. Arch Orthop Trauma Surg. 2005; 125(6):390–395. PMID:
15891920.


78. Willis-Owen CA, Daurka JS, Chen A, Lewis A. Bilateral femoral neck fractures due to transient osteoporosis of pregnancy: a case report. Cases J. 2008; 1(1):120. PMID:
18718014.



79. Van Veldhuizen PJ, Neff J, Murphey MD, Bodensteiner D, Skikne BS. Decreased fibrinolytic potential in patients with idiopathic avascular necrosis and transient osteoporosis of the hip. Am J Hematol. 1993; 44(4):243–248. PMID:
8237994.


80. Berger CE, Kluger R, Urban M, Kowalski J, Haas OA, Engel A. Elevated levels of lipoprotein(a) in familial bone marrow edema syndrome of the hip. Clin Orthop Relat Res. 2000; (377):126–131.

81. Koo KH, Ahn IO, Song HR, Kim SY, Jones JP Jr. Increased perfusion of the femoral head in transient bone marrow edema syndrome. Clin Orthop Relat Res. 2002; (402):171–175. PMID:
12218481.

82. Wilson AJ, Murphy WA, Hardy DC, Totty WG. Transient osteoporosis: transient bone marrow edema? Radiology. 1988; 167(3):757–760. PMID:
3363136.


83. Manara M, Varenna M. A clinical overview of bone marrow edema. Reumatismo. 2014; 66(2):184–196. PMID:
25069499.


84. Grottkau BE, Lin Y. Osteogenesis of adipose-derived stem cells. Bone Res. 2013; 1(2):133–145. PMID:
26273498.



85. Camp JF, Colwell CW Jr. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am. 1986; 68(9):1313–1319. PMID:
3782202.


86. Kiaer T, Pedersen NW, Kristensen KD, Starklint H. Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. J Bone Joint Surg Br. 1990; 72(6):1023–1030. PMID:
2246284.


87. Learmonth ID, Maloon S, Dall G. Core decompression for early atraumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 1990; 72(3):387–390. PMID:
2341433.


88. Downey DJ, Simkin PA, Taggart R. The effect of compressive loading on intraosseous pressure in the femoral head in vitro. J Bone Joint Surg Am. 1988; 70(6):871–877. PMID:
3392085.


89. Goddard NJ, Gosling PT. Intra-articular fluid pressure and pain in osteoarthritis of the hip. J Bone Joint Surg Br. 1988; 70(1):52–55. PMID:
3339061.


90. Lee YJ, Cui Q, Koo KH. Is there a role of pharmacological treatments in the prevention or treatment of osteonecrosis of the femoral head?: a systematic review. J Bone Metab. 2019; 26(1):13–18. PMID:
30899719.



91. Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012; 27(5):679–686. PMID:
22000577.

92. Tabatabaee RM, Saberi S, Parvizi J, Mortazavi SM, Farzan M. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015; 30(9):Suppl. 11–15. PMID:
26143238.


93. Goodman SB. The biological basis for concentrated iliac crest aspirate to enhance core decompression in the treatment of osteonecrosis. Int Orthop. 2018; 42(7):1705–1709. PMID:
29435623.


94. Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002; (405):14–23.

95. Hernigou P, Dubory A, Homma Y, Guissou I, Flouzat Lachaniette CH, Chevallier N, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018; 42(7):1639–1649. PMID:
29744647.

96. Houdek MT, Wyles CC, Collins MS, Howe BM, Terzic A, Behfar A, et al. Stem cells combined with platelet-rich plasma effectively treat corticosteroid-induced osteonecrosis of the hip: a prospective study. Clin Orthop Relat Res. 2018; 476(2):388–397. PMID:
29529674.



97. Shim KB, Kwon DS, Oh SJ, Kang JS, Moon KH. The efficacy of core decompression for treating avascular necrosis of the femoral head. J Korean Hip Soc. 2009; 21(3):219–225.

98. Yoon BH, Lee YK, Kim KC, Ha YC, Koo KH. No differences in the efficacy among various core decompression modalities and non-operative treatment: a network meta-analysis. Int Orthop. 2018; 42(12):2737–2743. PMID:
29855682.


99. Lee SH, Sim GB, Lee JB, Kim WK. Mid-term results of autologous bone marrow transplantation in osteonecrosis of the femoral head. Hip Pelvis. 2014; 26(1):7–13.





 PDF
PDF Citation
Citation Print
Print



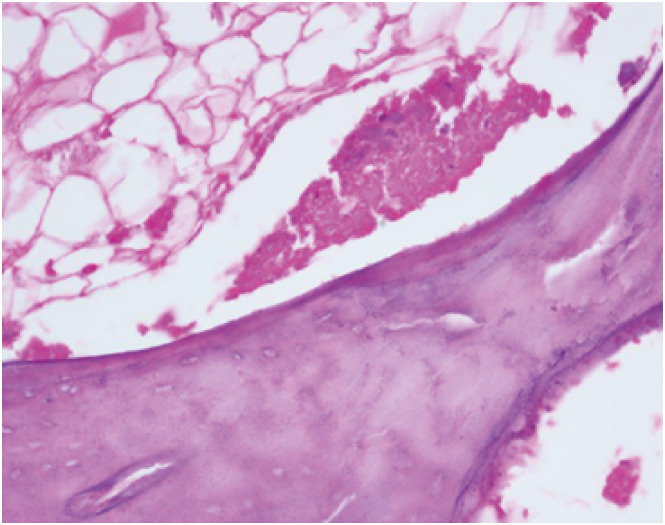
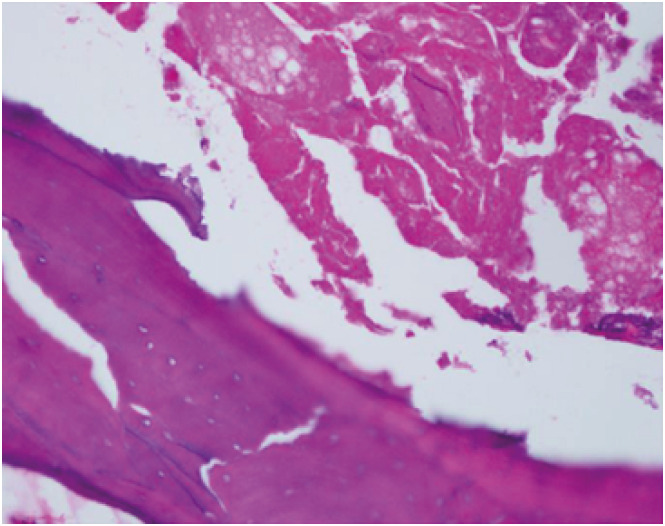
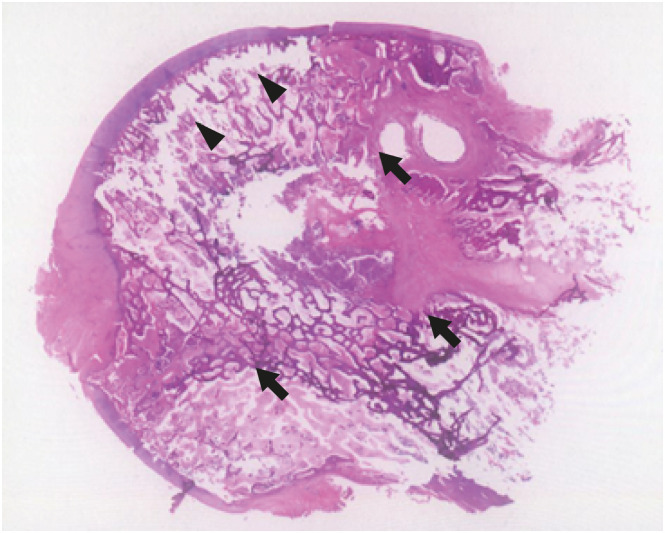
 XML Download
XML Download