INTRODUCTION
Kawasaki disease (KD) is an acute, systemic vasculitis that affects infants and young children, mainly children under the age of 5 years. It is characterized by prolonged fever that is unresponsive to antibiotics; polymorphous skin rash; erythema of the oral mucosa, lips, and tongue; erythema of the palms and soles; bilateral conjunctival injection; and cervical lymphadenopathy.
1) The primary pathological changes associated with KD include inflammation of medium-sized muscular arteries throughout the body, particularly the coronary arteries, and possible formation of coronary artery aneurysms.
2)3) High-dose intravenous immunoglobulin (IVIG) (2 g/kg over 12 hours) together with aspirin effectively abrogate the fever and systemic inflammation, and reduce the rate of aneurysm to less than 5% of cases.
4) Although the pathogenesis of KD remains unknown, it is thought to be due to an abnormal immune reaction triggered by unknown infectious agents in genetically susceptible individuals.
5)
Recent genome-wide association studies (GWAS) identified several genes related to B cell signaling, including
BLK,
CD40,
FCGR2A, and
BCL2L11,
6)7)8)9) as susceptibility genes for KD. Farh et al.
10) conducted bioinformatics analyses integrating published GWAS results and information about gene expression, expression quantitative trait loci, and epigenetic information, which suggested the importance of B cells in KD pathogenesis.
11) Thus, the data imply that B cells may play an important role in the pathogenesis of KD. Immunoglobulins (Igs) play key roles in B cell development and function. In this study, we measured Ig levels (IgG, IgA, IgM, and IgE) during the clinical course of KD and examined the effects of each Ig isotype level on inflammatory laboratory data and clinical outcomes (i.e., coronary artery lesions [CALs] and non-responsiveness to IVIG).
DISCUSSION
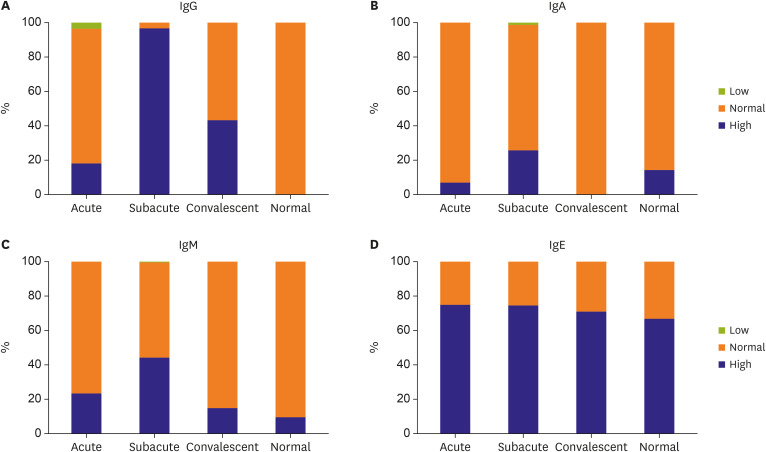
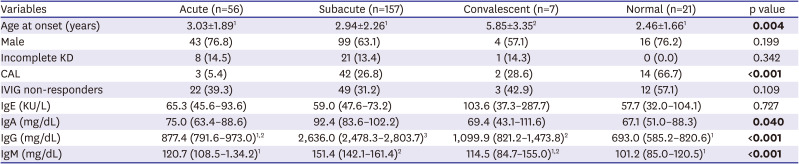
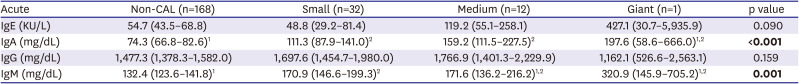
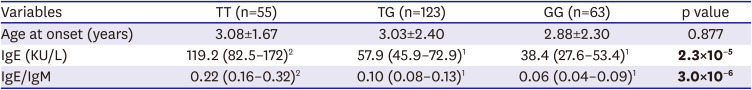
The data presented herein show that 74% of patients with KD had elevated IgE levels, and that the ACOXL-BCL2L11 locus is associated significantly with IgE levels and IgE isotype switching. However, elevated IgE levels in patients with KD were not associated with inflammatory data or with clinical outcomes. By contrast, high levels of IgA and IgM were significantly associated with development of CALs.
High IgE levels during the acute phase are a characteristic of KD.
17)18) This study also observed elevated IgE levels during all clinical phases of KD (compared with reference IgE values). Compared with controls, children with KD have a significantly higher incidence of allergic diseases associated with high IgE levels, including asthma, atopic dermatitis, and allergic rhinitis.
19)20) Wei et al.
21) also reported that children with allergic diseases are at high risk of KD, with the following adjusted ORs for having KD: 1.82 for urticaria, 1.44 for allergic rhinitis, and 1.22 for atopic dermatitis. All of these data suggest that KD tends to be associated with allergic diseases, and that both KD and allergic diseases share a common B cell-mediated pathogenesis. We also found that IgE levels were not associated with inflammatory laboratory data or with clinical outcome; the exception was that the incidence of complete KD was slightly higher in high IgE group (90.4% with complete KD vs. 79.4% in the normal IgE group; p=0.040). Therefore, the results indicate that elevated IgE levels in patients with KD may not affect the severity of inflammation or the pathogenesis of KD.
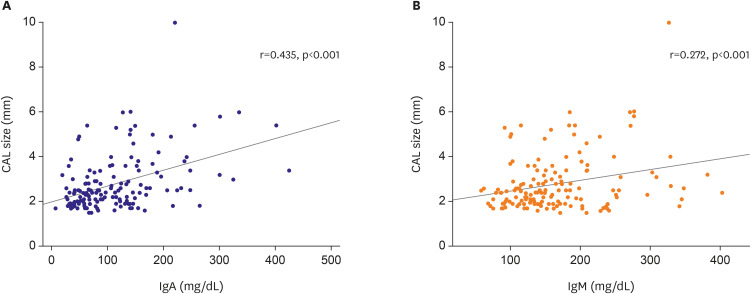
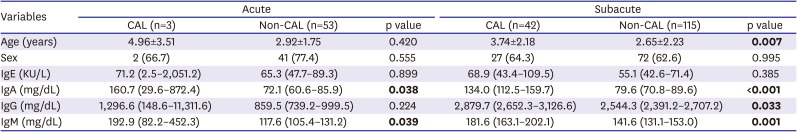
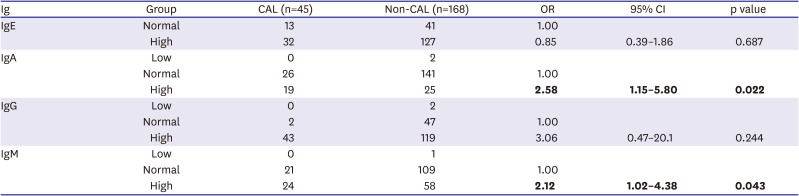
In this study, we found no significant difference between IgE and IgG levels and the clinical outcome of KD. However, IgA and IgM levels in the CAL group were significantly higher than those in the non-CAL group, and correlated with the severity of CALs. In particular, high IgA was much more significantly associated with CALs than IgM. For example, a larger effect size and significance was observed for IgA than for IgM when comparing the CAL group with the non-CAL group (
Tables 2 and
4). Moreover, a higher correlation was observed between CAL size and Ig levels for IgA than for IgM (r=0.435, p<0.001 for IgA; r=0.272, p<0.001;
Figure 2). Furthermore, high IgA levels were significantly associated with CALs (44.7% vs. 20.8% in the normal IgA group, p<0.01;
Supplementary Table 4), whereas high IgM levels were not (30.6% vs. 22.6% in the normal IgM group, p=0.332;
Supplementary Table 5). All of these results indicate that high IgA levels are superior to high IgM levels as a prognostic marker for predicting CAL risk in KD. Sawaji et al.
22) showed that IgG, IgM, and IgA z-scores in the CAL group (n=20) were significantly lower than those in the non-CAL group (n=68), and that a low IgG z-score was a risk factor for CALs. However, Yanagimoto et al.
23) reported no difference in IgG, IgA, or IgM z-scores in patients with and without CALs (16 cases with CAL vs. 181 cases without CALs). However, these 2 studies examined a very small number of samples obtained before IVIG treatment. Ding et al.
3) also reported no difference in IgA, IgG, and IgM levels between the CAL and non-CAL groups in a Chinese population. All of these results are different from our own. However, none of these previous studies are conclusive due to small sample sizes, different study populations, different measurement time points, and differences in data analysis methods. Further investigations are needed to identify true differences. Recent studies suggest that the IgA response in KD may be an important contributor to vasculitis linked to cardiovascular inflammation. Noval Rivas et al.
24) revealed that vascular inflammation is associated with intestinal permeability and elevated circulating secretory IgA (sIgA) levels in patients with KD, as well as with elevated sIgA and IgA deposition in vascular tissues in a mouse model of KD vasculitis. Furthermore, a high number of infiltrating oligoclonal IgA plasma cells were detected in inflammatory tissues of patients with KD.
25)26) These results suggest that IgA plays an important role in mediating vascular damage in KD.
We observed elevated levels of IgA, IgM, and IgG during the subacute phase of KD, and constitutively elevated IgE levels during the entire clinical course of KD. However, GWAS suggested that none of the loci associated with Ig levels or specific Ig isotype switching in patients with KD were associated with known loci for Ig levels or KD susceptibility; the exception was the
ACOXL-BCL2L11 locus. SNPs located in the
ACOXL gene were significantly associated with both IgE levels and IgE isotype switching (
Supplementary Table 11).
ACOXL, which encodes acyl-CoA oxidase-like protein, participates in fatty acid β-oxidation, fatty acid metabolic processes, and oxidation/reduction. Missense variant rs1554005 in the
ACOXL gene is associated with IgG isotype switching.
27) Recent GWAS has implicated the
ACOXL gene and its neighboring
BCL2L11 gene, which is located 2.4 kb downstream, in chronic lymphocytic leukemia.
28) The
BCL2L11 gene is also associated with KD.
9) In addition, rs1448187 in the
BCL2L11 locus is associated with elevated levels of IgA and IgG.
27) Wood et al.
29) reported that upon infection by EBV,
BCL2L11 was repressed by the EBV repressors EBNA3A and EBNA3C through inactivation of multiple enhancers in the regulatory hub encompassing the
ACOXL gene. Therefore, even if the
ACOXL gene SNP associated with IgE levels is not linked to the
BCL2L11 gene SNP associated with KD susceptibility, the
ACOXL locus may function as an enhancer that controls expression of
BCL2L11 in human B cells (
Supplementary Figure 1).
In this study, we demonstrated that high IgA levels may be a prognostic marker for predicting CAL risk in KD; however, the study has some limitations, including lack of healthy control Ig data, no continuous measurement of Ig concentrations in the same patients during all clinical phases, no information on the effect of IVIG infusion on the change of IgA and IgM levels, and no replication studies to confirm the GWAS results for Ig levels and Ig isotype switching. Therefore, further research is needed to overcome these limitations.
In summary, we found that the elevated IgE levels during all clinical phases are characteristic of KD, and we identified the ACOXL locus as being associated with IgE and IgE isotype switching in patients with KD. However, elevated IgE levels in patients with KD are not associated with clinical outcome. By contrast, IgA levels were significantly associated with development of CALs in KD.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download