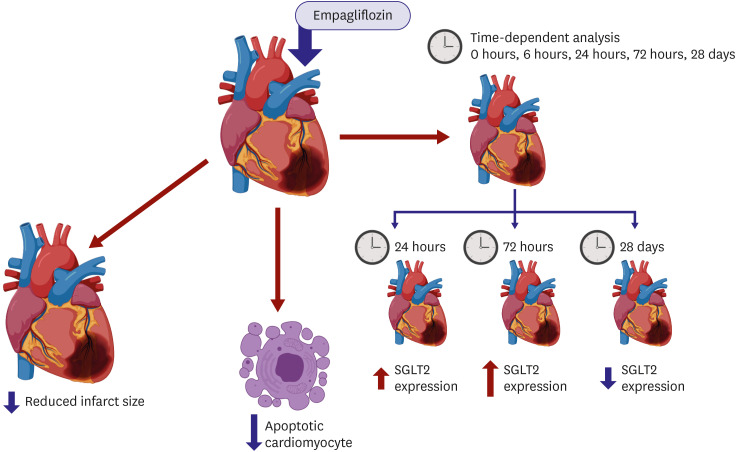
 | Figure 1Proposed action of SGLT2 inhibition on ischemic heart. The study of Lee et al.10) tested and determined that SGLT2 inhibition directly acts on the heart based on minimizing of infarcted region and apoptotic heart cells and time-dependent action (image was created using https://biorender.com/).SGLT2 = sodium glucose co-transporter 2.
|
Type 2 diabetes mellitus (T2DM) studies and its drug development aim to improve glycaemic control as well as mitigate the cardiovascular and renal risks and diseases associated with T2DM.1) The introduction of sodium glucose co-transporter 2 (SGLT2) inhibitors provides new insight and avenues in T2DM patients' health management, but this also presents treatment modes for the fields of nephrology and, in our journal's interest, for the field of cardiology.
Over the years, cardiovascular disease related with T2DM has become a prevalent health issue worldwide. T2DM morbidity and mortality have largely been associated with cardiovascular disease that is followed by subsequent heart failure seen in patients.2) In addition to this, T2DM has also been found to cause diabetic kidney disease. Patients afflicted with T2DM also experience renal disease that exacerbates the incidence of cardiovascular disease due to the ineffective action of the kidney in terms of glucose excretion.3)
The belief that cardiovascular benefits were indirectly related was due to SGLT2 transporters found largely in the kidney, at the proximal tubules. Due to the inhibition of SGLT2, glucose reabsorption is limited and glucose excretion is increased.1) This then lowers the glycated hemoglobin plasma levels that further improve conditions in terms of the body weight, insulin and blood pressure. Prevention and reduction of heart failure hospitalization was an unexpected cardiovascular benefit from SGLT2 inhibitors.1)2)3) Meta-analysis by Zelniker et al.4) presented that efficiency of SGLT2 inhibitors in preventing heart failure was observed from patients with renal disease. This then established that the condition of the kidney may contribute in either promoting or preventing heart failure.
With this in mind, large-scale clinical studies were launched to determine the safety, benefits and effects of SGLT2 inhibitors to be used as cardioprotective drugs. These mandated clinical trials brought to the forefront how SGLT2 inhibitors rapidly reduced renal and, what was assumed indirectly, cardiovascular adverse events. The clinical trials of interest such as BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, CANagliflozin cardioVascular Assessment Study program and Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction largely identified patients with heart failure in their medical histories.5)6)7) There was a pronounced speed in the reduction and recovery of patients from cardiovascular diseases with hospitalisations due to stroke, heart failure, myocardial infarction (MI), and even cardiovascular-related mortality. It is noted by Cowie and Fisher, however, that the cardiovascular benefits detected in the EMPA-REG OUTCOME study appears to be independent of the primary action of SGLT2 inhibitors which is reduction in glucose uptake.8) In another trial, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure, patients without T2DM preconditions but were in heart failure saw an increased cardiovascular performance following drug treatment.9) However, it is with caution that SGLT2 inhibitors are recommended as cardioprotective drugs given that most of its action is focused in the kidneys. It has been inferred that SGLT2 inhibitors indirectly benefit the heart given the absence of SGLT2 in the heart per se. Lee et al.10) asserts, however, that SGLT2 may be present in the heart but only for a short time and during cardiovascular disease conditions such as MI.
In this novel study, the results contribute to the body of knowledge about the direct action of SGLT2 inhibitors, specifically empagliflozin (EMPA), on cardiovascular disease and subsequent MI. The paper inferred and provided evidence that SGLT2 expression may be dependent on several factors such as the pre-existing condition of the heart and the time duration of SGLT2 (Figure 1). This study used a mouse model for non-diabetic MI and used nuclear magnetic resonance imaging to determine the metabolic effects of SGLT2 inhibitor on the heart. Further evaluation of SGLT2 presence in the heart was done through infarct and apoptosis analysis, histological and immunofluorescence imaging, and western blot analysis.
Lee et al.10) formulated the following hypotheses in relation to SGLT2 inhibitor, EMPA, and its direct action on the heart, counterintuitive to what is believed to be an indirect effect by the largely renal-centered action of SGLT2 inhibitors due to the presence of SGLT2 in the nephrons. SGLT, in general, is said to be absent in heart tissue but this research asserts its presence. The first hypothesis is that the presence or absence of SGLT in the system is circumstantial given that SGLT1 expression relies largely on the stress or stimuli received in the system. Second, SGLT2 is time-dependent after MI. Results coincide with these inferences given that SGLT2 expression was localized only in the injured area and at a certain time frame only and with EMPA delaying the action of SGLT2. SGLT2 expression is also related to cardiovascular disease-related proteins that were apparent following western blot analysis such as transforming growth factor beta 1, hypoxia-inducible factor 1 alpha, and SMAD2 that were all expressed dependent on the time of infarction which decreased upon EMPA treatment. The researchers also assert that EMPA treatment may also aid in modification of metabolites when under MI conditions. A shift in the metabolism may suggest that EMPA pretreatment redirects how adenosine triphosphate is produced and thus, may improve the heart condition as compared to when under ischemia. This then provides evidence to the direct effect of SGLT2 inhibitors to the heart and not just through the renal pathway.
However, there is still the question of what cells specifically express SGLT2 in the heart. This study was not able to provide concrete evidence as to whether it is convincingly the heart cells or transient cell types such as inflammatory cells that carried SGLT2 expression. Yet, it must not be set aside that this research sets a precedent on the direct effect of SGLT2 inhibition in the heart. Solid evidence has been provided herein that can be used for further SGLT2 inhibition-related cardiovascular disease research.
Currently, there is a movement towards determining the mechanisms underlying SGLT2 inhibition in the heart especially with its obvious benefits towards cardiovascular disease. If we are able to concretely determine the pathway of SGLT2 inhibition, there is no doubt that we can only move deeper and wider towards mitigating morbidity and mortality from cardiovascular disease.
Notes
Funding: This research was supported by the Basic Research Lab Program (NRF2020R1A4A1018943) and the Basic Science Research Program (2018R1A2A3074998) through the National Research Foundation of Korea funded by the Ministry of Science and ICT, Korea
The contents of the report are the author's own views and do not necessarily reflect the views of the Korean Circulation Journal.
Go to : 
References
1. Kim YS, Ahn Y. Benefits of SGLT2 inhibitor: preventing heart failure and beyond. Korean Circ J. 2019; 49:1196–1198. PMID: 31642218.


2. Lehrke M. SGLT2 inhibition: changing what fuels the heart. J Am Coll Cardiol. 2019; 73:1945–1947. PMID: 30999997.

3. Hwang J, Kim DB, Park HJ. Off-target effects of sodium-glucose cotransporter 2 (SGLT-2) inhibitor in cardiovascular disease. Korean Circ J. 2020; 50:458–460. PMID: 32216177.


4. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. PMID: 30424892.
5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID: 26378978.

6. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. PMID: 28605608.

7. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID: 30415602.

8. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020; 17:761–772. PMID: 32665641.

9. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008. PMID: 31535829.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download