1. Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020; 106:1380–1386. PMID:
32522821.

2. Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007; 356:1503–1516. PMID:
17387127.

3. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020; 382:1395–1407. PMID:
32227755.


4. Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008; 117:1283–1291. PMID:
18268144.

5. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40:87–165. PMID:
30165437.

6. Jeremias A, Maehara A, Généreux P, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014; 63:1253–1261. PMID:
24211503.

7. Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR Study (joined coronary pressure and flow analysis to determine diagnostic characteristics of basal and hyperemic indices of functional lesion severity-coronary flow reserve). Circ Cardiovasc Interv. 2014; 7:492–502. PMID:
24987048.

8. Sen S, Asrress KN, Nijjer S, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (classification accuracy of pressure-only ratios against indices using flow study). J Am Coll Cardiol. 2013; 61:1409–1420. PMID:
23500218.

9. Svanerud J, Ahn JM, Jeremias A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the resting full-cycle ratio (VALIDATE RFR) study. EuroIntervention. 2018; 14:806–814. PMID:
29790478.

10. Van't Veer M, Pijls NHJ, Hennigan B, et al. Comparison of different diastolic resting indexes to iFR: are they all equal? J Am Coll Cardiol. 2017; 70:3088–3096. PMID:
29268922.

11. Lee JM, Choi KH, Park J, et al. Physiological and clinical assessment of resting physiological indexes. Circulation. 2019; 139:889–900. PMID:
30586749.

12. Hwang D, Lee JM, Yang S, et al. Role of post-stent physiological assessment in a risk prediction model after coronary stent implantation. JACC Cardiovasc Interv. 2020; 13:1639–1650. PMID:
32703590.

13. Johnson NP, Tóth GG, Lai D, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014; 64:1641–1654. PMID:
25323250.

14. Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: a systematic review and meta-analysis. Am Heart J. 2017; 183:1–9. PMID:
27979031.
15. Hannan EL, Racz M, Holmes DR, et al. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation. 2006; 113:2406–2412. PMID:
16702469.

16. Rosner GF, Kirtane AJ, Genereux P, et al. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: the acute catheterization and urgent intervention triage strategy (ACUITY) trial. Circulation. 2012; 125:2613–2620. PMID:
22550156.

17. Farooq V, Serruys PW, Bourantas CV, et al. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. 2013; 128:141–151. PMID:
23766350.

18. Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (synergy between percutaneous coronary intervention with taxus and cardiac surgery) trial. J Am Coll Cardiol. 2013; 61:282–294. PMID:
23265332.

19. Takano Y, Yeatman LA, Higgins JR, et al. Optimizing stent expansion with new stent delivery systems. J Am Coll Cardiol. 2001; 38:1622–1627. PMID:
11704372.

20. Bertrand OF, De Larochellière R, Joyal M, Bonan R, Mongrain R, Tardif JC. Incidence of stent under-deployment as a cause of in-stent restenosis in long stents. Int J Cardiovasc Imaging. 2004; 20:279–284. PMID:
15529909.

21. Im E, Kim BK, Ko YG, et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014; 7:88–96. PMID:
24425586.

22. Kawamori H, Shite J, Shinke T, et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur Heart J Cardiovasc Imaging. 2013; 14:865–875. PMID:
23291393.


23. Lee CH, Hur SH. Optimization of Percutaneous Coronary Intervention Using Optical Coherence Tomography. Korean Circ J. 2019; 49:771–793. PMID:
31456372.


24. Nagaoka H, Iizuka T, Kubota S, et al. Redistribution in thallium-201 myocardial imaging soon after successful coronary stenting--tomographic evaluation during coronary hyperemia induced by adenosine. Jpn Circ J. 1998; 62:160–166. PMID:
9583440.

25. Rodés-Cabau J, Candell-Riera J, Domingo E, et al. Frequency and clinical significance of myocardial ischemia detected early after coronary stent implantation. J Nucl Med. 2001; 42:1768–1772. PMID:
11752071.
26. Kim J, Lee JM, Choi SH, et al. Comparison of exercise performance and clinical outcome between functional complete and incomplete revascularization. Korean Circ J. 2020; 50:406–417. PMID:
32096361.


27. Wolfrum M, De Maria GL, Benenati S, et al. What are the causes of a suboptimal FFR after coronary stent deployment? Insights from a consecutive series using OCT imaging. EuroIntervention. 2018; 14:e1324–31. PMID:
29784630.
28. Hanekamp CE, Koolen JJ, Pijls NH, Michels HR, Bonnier HJ. Comparison of quantitative coronary angiography, intravascular ultrasound, and coronary pressure measurement to assess optimum stent deployment. Circulation. 1999; 99:1015–1021. PMID:
10051294.

29. Ito T, Tani T, Fujita H, Ohte N. Relationship between fractional flow reserve and residual plaque volume and clinical outcomes after optimal drug-eluting stent implantation: insight from intravascular ultrasound volumetric analysis. Int J Cardiol. 2014; 176:399–404. PMID:
25125008.

30. Bech GJ, Pijls NH, De Bruyne B, et al. Usefulness of fractional flow reserve to predict clinical outcome after balloon angioplasty. Circulation. 1999; 99:883–888. PMID:
10027810.

31. Nam CW, Hur SH, Cho YK, et al. Relation of fractional flow reserve after drug-eluting stent implantation to one-year outcomes. Am J Cardiol. 2011; 107:1763–1767. PMID:
21481828.

32. Leesar MA, Satran A, Yalamanchili V, Helmy T, Abdul-Waheed M, Wongpraparut N. The impact of fractional flow reserve measurement on clinical outcomes after transradial coronary stenting. EuroIntervention. 2011; 7:917–923. PMID:
22157476.

33. Matsuo A, Fujita H, Tanigaki T, et al. Clinical implications of coronary pressure measurement after stent implantation. Cardiovasc Interv Ther. 2013; 28:170–177. PMID:
23161151.

34. Doh JH, Nam CW, Koo BK, et al. Clinical relevance of poststent fractional flow reserve after drug-eluting stent implantation. J Invasive Cardiol. 2015; 27:346–351. PMID:
26232010.

35. Reith S, Battermann S, Hellmich M, Marx N, Burgmaier M. Correlation between OCT-derived intrastent dimensions and fractional flow reserve measurements after coronary stent implantation and impact on clinical outcome. J Invasive Cardiol. 2015; 27:222–228. PMID:
25929298.

36. Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing post-intervention fractional flow reserve to optimize acute results and the relationship to long-term outcomes. JACC Cardiovasc Interv. 2016; 9:1022–1031. PMID:
27198682.

37. Kasula S, Agarwal SK, Hacioglu Y, et al. Clinical and prognostic value of poststenting fractional flow reserve in acute coronary syndromes. Heart. 2016; 102:1988–1994. PMID:
27492942.

38. Li SJ, Ge Z, Kan J, et al. Cutoff value and long-term prediction of clinical events by FFR measured immediately after implantation of a drug-eluting stent in patients with coronary artery disease: 1- to 3-year results from the DKCRUSH VII registry study. JACC Cardiovasc Interv. 2017; 10:986–995. PMID:
28456699.

39. Piroth Z, Toth GG, Tonino PAL, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv. 2017; 10.
40. Lee JM, Hwang D, Choi KH, et al. Prognostic implications of relative increase and final fractional flow reserve in patients with stent implantation. JACC Cardiovasc Interv. 2018; 11:2099–2109. PMID:
30336814.

41. Hwang D, Lee JM, Lee HJ, et al. Influence of target vessel on prognostic relevance of fractional flow reserve after coronary stenting. EuroIntervention. 2019; 15:457–464. PMID:
30561367.

42. Hakeem A, Ghosh B, Shah K, et al. Incremental prognostic value of post-intervention Pd/Pa in patients undergoing ischemia-driven percutaneous coronary intervention. JACC Cardiovasc Interv. 2019; 12:2002–2014. PMID:
31648762.

43. van Bommel RJ, Masdjedi K, Diletti R, et al. Routine fractional flow reserve measurement after percutaneous coronary intervention. Circ Cardiovasc Interv. 2019; 12:e007428. PMID:
31018666.
44. Azzalini L, Poletti E, Demir OM, et al. Impact of post-percutaneous coronary intervention fractional flow reserve measurement on procedural management and clinical outcomes: the REPEAT-FFR study. J Invasive Cardiol. 2019; 31:229–234. PMID:
31199348.

45. Hoshino M, Kanaji Y, Hamaya R, et al. Prognostic value of post-intervention fractional flow reserve after intravascular ultrasound-guided second-generation drug-eluting coronary stenting. EuroIntervention. 2019; 15:e779–87. PMID:
31012854.
46. Shin D, Lee SH, Lee JM, et al. Prognostic implications of post-intervention resting Pd/Pa and fractional flow reserve in patients with stent implantation. JACC Cardiovasc Interv. 2020; 13:1920–1933. PMID:
32819481.

47. Pijls NH, Klauss V, Siebert U, et al. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation. 2002; 105:2950–2954. PMID:
12081986.

48. Jeremias A, Davies JE, Maehara A, et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI study. JACC Cardiovasc Interv. 2019; 12:1991–2001. PMID:
31648761.

49. Hamaya R, Mittleman MA, Hoshino M, et al. Prognostic value of pre-revascularization fractional flow reserve mediated by the post-revascularization level: a causal mediation analysis. JAMA Netw Open. 2020; 3:e2018162. PMID:
32997128.
50. De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart. 2008; 94:949–959. PMID:
18552231.

51. Murai T, Lee T, Yonetsu T, Isobe M, Kakuta T. Influence of microvascular resistance on fractional flow reserve after successful percutaneous coronary intervention. Catheter Cardiovasc Interv. 2015; 85:585–592. PMID:
24678006.

52. Biscaglia S, Tebaldi M, Brugaletta S, et al. Prognostic value of QFR measured immediately after successful stent implantation: the international multicenter prospective HAWKEYE study. JACC Cardiovasc Interv. 2019; 12:2079–2088. PMID:
31563688.

53. Kikuta Y, Cook CM, Sharp AS, et al. Pre-angioplasty instantaneous wave-free ratio pullback predicts hemodynamic outcome in humans with coronary artery disease: primary results of the international multicenter iFR GRADIENT registry. JACC Cardiovasc Interv. 2018; 11:757–767. PMID:
29673507.

54. Frimerman A, Abu-Fane R, Levi Y, et al. Novel method for real-time coregistration of coronary physiology and angiography by iFR. JACC Cardiovasc Interv. 2019; 12:692–694. PMID:
30947947.

55. Matsuo A, Kasahara T, Ariyoshi M, et al. Utility of angiography-physiology coregistration maps during percutaneous coronary intervention in clinical practice. Cardiovasc Interv Ther. 2020; [Epub ahead of print].
56. Omori H, Kawase Y, Mizukami T, et al. Comparisons of nonhyperemic pressure ratios: predicting functional results of coronary revascularization using longitudinal vessel interrogation. JACC Cardiovasc Interv. 2020; 13:2688–2698. PMID:
33129819.

57. Völz S, Dworeck C, Redfors B, et al. Survival of patients with angina pectoris undergoing percutaneous coronary intervention with intracoronary pressure wire guidance. J Am Coll Cardiol. 2020; 75:2785–2799. PMID:
32498806.

58. Lee SH, Shin D, Lee JM, et al. Automated algorithm using pre-intervention fractional flow reserve pullback curve to predict post-intervention physiological results. JACC Cardiovasc Interv. 2020; 13:2670–2684. PMID:
33069650.

59. Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015; 36:3359–3367. PMID:
26330417.


60. Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol. 2014; 63:1145–1155. PMID:
24486266.

61. Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012; 308:1237–1245. PMID:
22922562.


62. Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol. 2011; 58:1989–1997. PMID:
22032711.

63. Kay FU, Canan A, Abbara S. Future directions in coronary CT angiography: CT-fractional flow reserve, plaque vulnerability, and quantitative plaque assessment. Korean Circ J. 2020; 50:185–202. PMID:
31960635.

64. Kim KH, Doh JH, Koo BK, et al. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv. 2014; 7:72–78. PMID:
24332418.
65. Bom MJ, Schumacher SP, Driessen RS, et al. Non-invasive procedural planning using computed tomography-derived fractional flow reserve. Catheter Cardiovasc Interv. 2020; [Epub ahead of print].
66. Tu S, Westra J, Adjedj J, et al. Fractional flow reserve in clinical practice: from wire-based invasive measurement to image-based computation. Eur Heart J. 2020; 41:3271–3279. PMID:
31886479.

67. Kogame N, Takahashi K, Tomaniak M, et al. Clinical implication of quantitative flow ratio after percutaneous coronary intervention for 3-vessel disease. JACC Cardiovasc Interv. 2019; 12:2064–2075. PMID:
31563682.

68. Rubimbura V, Guillon B, Fournier S, et al. Quantitative flow ratio virtual stenting and post stenting correlations to post stenting fractional flow reserve measurements from the DOCTORS (does optical coherence tomography optimize results of stenting) study population. Catheter Cardiovasc Interv. 2020; 96:1145–1153. PMID:
31763775.

69. Dai N, Hwang D, Lee JM, et al. Feasibility of quantitative flow ratio-derived pullback pressure gradient index and its impact on diagnostic performance. JACC Cardiovasc Interv. 2021; 14:353–355. PMID:
33541549.

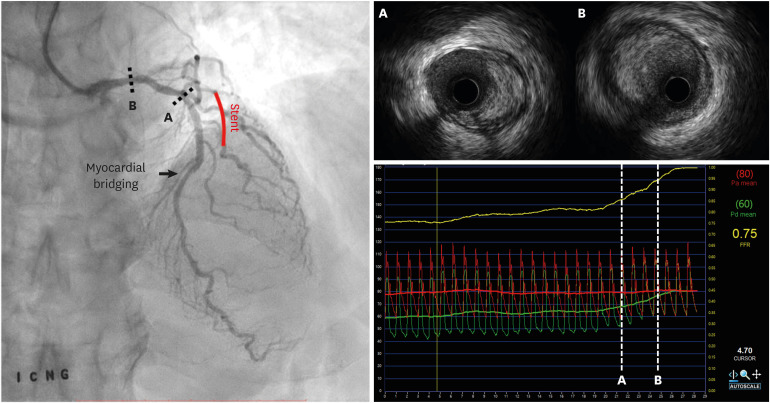
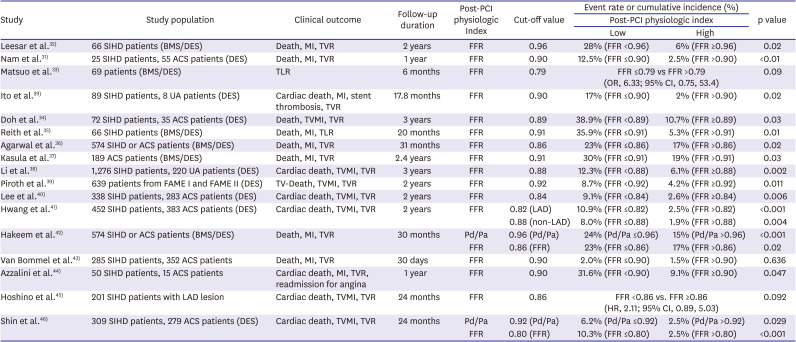

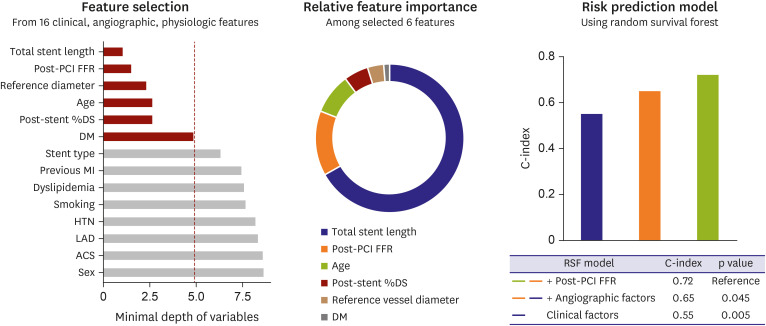





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download