METHODS
In Korea, SAPIEN 3 valves (Edwards Lifescience, Irvine, CA, USA) were first used in April 2016, whereas Evolut R and PRO valves (Medtronitc, Minneapolis, MN, USA) were first used in August 2015 and January 2019, respectively. Between April 2016 and March 2020, 271 patients who underwent TAVR for symptomatic severe aortic stenosis at our institute were initially identified. Among them, a total of 70 consecutive patients with small aortic annulus were included in the analysis. Inclusion criteria were small aortic annulus defined as an annulus exhibiting a mean diameter of ≤23 mm or minimal diameter of ≤21 mm on computed tomography, and TAVR with either a self-expanding 26 mm Evolut R or PRO valve or a balloon-expandable 23 mm SAPIEN 3 valve.
9) Forty-five patients underwent TAVR with an Evolut R (n=37) or a PRO (n=8) valve while 25 patients had a SAPIEN 3 valve. This study complied with the principles of the Declaration of Helsinki 2013, and the study protocol was approved by Institutional Review Board of our institute. Written informed consent was obtained from all patients. For every patient, a multidisciplinary heart team including interventional cardiologists, imaging cardiologists, cardiothoracic surgeons, and anesthesiologists made decisions for eligibility for TAVR, choice of vascular approach, and type of anesthesia. The choice of transcatheter aortic valve type was at the discretion of the operating interventional cardiologists. Implantation technique for both valves has been described previously.
10)11) The rate of permanent pacemaker implantation, early safety outcomes (at 30 days) and 1-year clinical outcomes according to the Valve Academic Research Consortium (VARC)-2 consensus, and echocardiographic hemodynamic outcomes were analyzed.
12) The early safety outcomes included all-cause mortality, all stroke, life-threatening bleeding, acute kidney injury stage 2 or 3, coronary artery obstruction requiring intervention, major vascular complication, and valve-related dysfunction requiring repeat procedure. The hemodynamic outcomes based on transthoracic echocardiography included EOA, indexed EOA (iEOA), mean aortic valve gradient, and PPM. EOA was estimated with the continuity equation and indexed to body surface area (BSA) as calculated with the DuBois formula.
13) PPM was defined as moderate for post-procedural 0.65 cm
2/m
2< iEOA ≤0.85 cm
2/m
2 and severe for iEOA ≤0.65 cm
2/m
2 in accordance to the VARC-2 consensus.
12) In addition, doppler velocity index was calculated and paravalvular leak was assessed and classified as none/trace, mild, moderate, or severe.
12)
Statistical analysis
Continuous variables are reported as mean±standard deviation and were compared using Student's t-tests. Categorical variables are reported as number (percentage) and were compared using χ2 tests or Fisher's exact tests. Event rates at 1 year were estimated using Kaplan-Meier survival analysis and compared using log-rank tests. All tests were two-tailed, and a p<0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS, version 23.0 (IBM Corporation, Armonk, NY, USA).
Ethics statement
This study was approved by the Institutional Review Board at Severance Hospital of the Yonsei University Health System (1-2009-0018, 1-2011-0099).
RESULTS
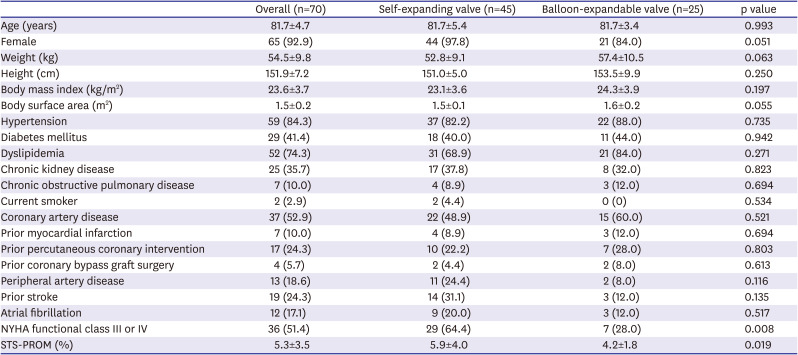
Of 271 patients who underwent TAVR for symptomatic severe aortic stenosis between April 2016 and March 2020, a total of 70 (25.8%) consecutive patients presented with small aortic annulus and received either a 26 mm Evolut R or PRO valve (n=45) or a 23 mm SAPIEN 3 valve (n=25). Baseline clinical characteristics are presented in
Table 1. Across the overall study population, mean age was 81.7±4.7 years old and the vast majority of patients were female (92.9%). Body mass index was 23.6±3.7 kg/m
2, BSA was 1.5±0.2 m
2, and 51.4% of patients were in New York Heart Association (NYHA) functional class III or IV. The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 5.3±3.5%. Baseline clinical characteristics between the self-expanding and balloon-expandable valve-treated groups were similar except for presence of NYHA functional class III or IV (64.4% vs. 28.0%, respectively, p=0.008) and the STS-PROM score (5.9±4.0% vs. 4.2±1.8%, respectively, p=0.019), which showed that the patients who underwent TAVR with a self-expanding valve had more severe symptoms of heart failure and higher operative risk. Medications at discharge are presented in
Supplementary Table 1.
Table 1
Baseline clinical characteristics
|
Overall (n=70) |
Self-expanding valve (n=45) |
Balloon-expandable valve (n=25) |
p value |
|
Age (years) |
81.7±4.7 |
81.7±5.4 |
81.7±3.4 |
0.993 |
|
Female |
65 (92.9) |
44 (97.8) |
21 (84.0) |
0.051 |
|
Weight (kg) |
54.5±9.8 |
52.8±9.1 |
57.4±10.5 |
0.063 |
|
Height (cm) |
151.9±7.2 |
151.0±5.0 |
153.5±9.9 |
0.250 |
|
Body mass index (kg/m2) |
23.6±3.7 |
23.1±3.6 |
24.3±3.9 |
0.197 |
|
Body surface area (m2) |
1.5±0.2 |
1.5±0.1 |
1.6±0.2 |
0.055 |
|
Hypertension |
59 (84.3) |
37 (82.2) |
22 (88.0) |
0.735 |
|
Diabetes mellitus |
29 (41.4) |
18 (40.0) |
11 (44.0) |
0.942 |
|
Dyslipidemia |
52 (74.3) |
31 (68.9) |
21 (84.0) |
0.271 |
|
Chronic kidney disease |
25 (35.7) |
17 (37.8) |
8 (32.0) |
0.823 |
|
Chronic obstructive pulmonary disease |
7 (10.0) |
4 (8.9) |
3 (12.0) |
0.694 |
|
Current smoker |
2 (2.9) |
2 (4.4) |
0 (0) |
0.534 |
|
Coronary artery disease |
37 (52.9) |
22 (48.9) |
15 (60.0) |
0.521 |
|
Prior myocardial infarction |
7 (10.0) |
4 (8.9) |
3 (12.0) |
0.694 |
|
Prior percutaneous coronary intervention |
17 (24.3) |
10 (22.2) |
7 (28.0) |
0.803 |
|
Prior coronary bypass graft surgery |
4 (5.7) |
2 (4.4) |
2 (8.0) |
0.613 |
|
Peripheral artery disease |
13 (18.6) |
11 (24.4) |
2 (8.0) |
0.116 |
|
Prior stroke |
19 (24.3) |
14 (31.1) |
3 (12.0) |
0.135 |
|
Atrial fibrillation |
12 (17.1) |
9 (20.0) |
3 (12.0) |
0.517 |
|
NYHA functional class III or IV |
36 (51.4) |
29 (64.4) |
7 (28.0) |
0.008 |
|
STS-PROM (%) |
5.3±3.5 |
5.9±4.0 |
4.2±1.8 |
0.019 |

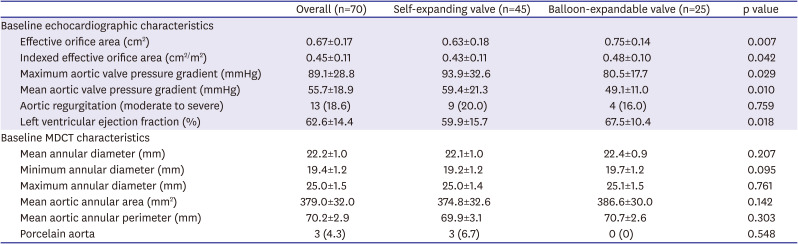
Baseline echocardiographic and computed tomographic characteristics are presented in
Table 2. Across the overall study population, EOA was 0.67±0.17 cm
2 and iEOA was 0.45±0.11 cm
2/m
2 by transthoracic echocardiography. In addition, mean annular diameter was 22.2±1.0 mm, minimum annular diameter was 19.4±1.2 mm, mean aortic annular area was 379.0±32.0 mm
2, and mean aortic annular perimeter was 70.2±2.9 mm by computed tomography. Baseline computed tomographic characteristics between the two groups were similar, whereas the patients who underwent TAVR with a self-expanding valve had more advanced features of aortic stenosis on echocardiography. To be specific, compared with the balloon-expandable valve-treated patients, the self-expanding valve-treated patients showed smaller EOA (0.75±0.14 vs. 0.63±0.18 cm
2, p=0.007) and iEOA (0.48±0.10 vs. 0.43±0.11 cm
2/m
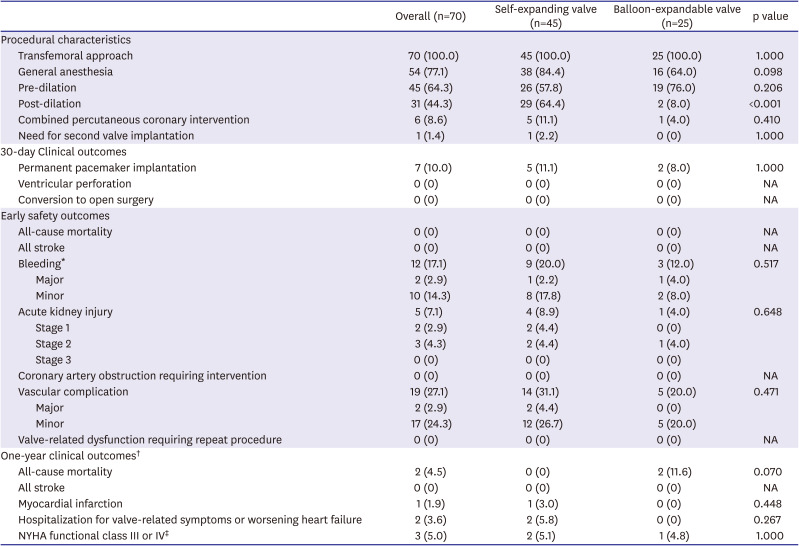
2, p=0.042). Procedural characteristics and 30-day clinical outcomes are presented in
Table 3. Procedural characteristics between the two groups were similar except for post-dilation, which was more frequently performed in the self-expanding valve-treated patients (64.4% vs. 8.0% in balloon-expandable valve-treated patients, p<0.001). The early safety outcomes within 30 days after TAVR were comparable between the two groups. At 1-year follow-up, no significant differences were observed in terms of all-cause mortality, stroke, myocardial infarction or hospitalization for valve-related symptoms or worsening heart failure between the two groups (
Table 3).
Table 2
Baseline echocardiographic and computed tomographic characteristics
|
Overall (n=70) |
Self-expanding valve (n=45) |
Balloon-expandable valve (n=25) |
p value |
|
Baseline echocardiographic characteristics |
|
|
|
|
|
Effective orifice area (cm2) |
0.67±0.17 |
0.63±0.18 |
0.75±0.14 |
0.007 |
|
Indexed effective orifice area (cm2/m2) |
0.45±0.11 |
0.43±0.11 |
0.48±0.10 |
0.042 |
|
Maximum aortic valve pressure gradient (mmHg) |
89.1±28.8 |
93.9±32.6 |
80.5±17.7 |
0.029 |
|
Mean aortic valve pressure gradient (mmHg) |
55.7±18.9 |
59.4±21.3 |
49.1±11.0 |
0.010 |
|
Aortic regurgitation (moderate to severe) |
13 (18.6) |
9 (20.0) |
4 (16.0) |
0.759 |
|
Left ventricular ejection fraction (%) |
62.6±14.4 |
59.9±15.7 |
67.5±10.4 |
0.018 |
|
Baseline MDCT characteristics |
|
|
|
|
|
Mean annular diameter (mm) |
22.2±1.0 |
22.1±1.0 |
22.4±0.9 |
0.207 |
|
Minimum annular diameter (mm) |
19.4±1.2 |
19.2±1.2 |
19.7±1.2 |
0.095 |
|
Maximum annular diameter (mm) |
25.0±1.5 |
25.0±1.4 |
25.1±1.5 |
0.761 |
|
Mean aortic annular area (mm2) |
379.0±32.0 |
374.8±32.6 |
386.6±30.0 |
0.142 |
|
Mean aortic annular perimeter (mm) |
70.2±2.9 |
69.9±3.1 |
70.7±2.6 |
0.303 |
|
Porcelain aorta |
3 (4.3) |
3 (6.7) |
0 (0) |
0.548 |

Table 3
Procedural characteristics and clinical outcomes
|
Overall (n=70) |
Self-expanding valve (n=45) |
Balloon-expandable valve (n=25) |
p value |
|
Procedural characteristics |
|
|
|
|
|
Transfemoral approach |
70 (100.0) |
45 (100.0) |
25 (100.0) |
1.000 |
|
General anesthesia |
54 (77.1) |
38 (84.4) |
16 (64.0) |
0.098 |
|
Pre-dilation |
45 (64.3) |
26 (57.8) |
19 (76.0) |
0.206 |
|
Post-dilation |
31 (44.3) |
29 (64.4) |
2 (8.0) |
<0.001 |
|
Combined percutaneous coronary intervention |
6 (8.6) |
5 (11.1) |
1 (4.0) |
0.410 |
|
Need for second valve implantation |
1 (1.4) |
1 (2.2) |
0 (0) |
1.000 |
|
30-day Clinical outcomes |
|
|
|
|
|
Permanent pacemaker implantation |
7 (10.0) |
5 (11.1) |
2 (8.0) |
1.000 |
|
Ventricular perforation |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Conversion to open surgery |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Early safety outcomes |
|
|
|
|
|
All-cause mortality |
0 (0) |
0 (0) |
0 (0) |
NA |
|
All stroke |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Bleeding*
|
12 (17.1) |
9 (20.0) |
3 (12.0) |
0.517 |
|
|
Major |
2 (2.9) |
1 (2.2) |
1 (4.0) |
|
|
Minor |
10 (14.3) |
8 (17.8) |
2 (8.0) |
|
Acute kidney injury |
5 (7.1) |
4 (8.9) |
1 (4.0) |
0.648 |
|
|
Stage 1 |
2 (2.9) |
2 (4.4) |
0 (0) |
|
|
Stage 2 |
3 (4.3) |
2 (4.4) |
1 (4.0) |
|
|
Stage 3 |
0 (0) |
0 (0) |
0 (0) |
|
Coronary artery obstruction requiring intervention |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Vascular complication |
19 (27.1) |
14 (31.1) |
5 (20.0) |
0.471 |
|
|
Major |
2 (2.9) |
2 (4.4) |
0 (0) |
|
|
Minor |
17 (24.3) |
12 (26.7) |
5 (20.0) |
|
Valve-related dysfunction requiring repeat procedure |
0 (0) |
0 (0) |
0 (0) |
NA |
|
One-year clinical outcomes†
|
|
|
|
|
|
All-cause mortality |
2 (4.5) |
0 (0) |
2 (11.6) |
0.070 |
|
All stroke |
0 (0) |
0 (0) |
0 (0) |
NA |
|
Myocardial infarction |
1 (1.9) |
1 (3.0) |
0 (0) |
0.448 |
|
Hospitalization for valve-related symptoms or worsening heart failure |
2 (3.6) |
2 (5.8) |
0 (0) |
0.267 |
|
NYHA functional class III or IV‡
|
3 (5.0) |
2 (5.1) |
1 (4.8) |
1.000 |

Hemodynamic outcomes by transthoracic echocardiography at discharge and 1-year follow-up are shown in
Table 4. At discharge, the self-expanding valve-treated patients presented significantly larger EOA (1.75±0.42 vs. 1.46±0.28 cm
2, p=0.002) and iEOA (1.18±0.28 vs. 0.95±0.21 cm
2/m
2, p=0.001) compared with the balloon-expandable valve-treated patients (
Figure 1A and B). Consequently, the incidence of PPM was significantly less frequent in the self-expanding valve-treated patients (8.9% vs. 36.0% in balloon-expandable valve-treated patients, p=0.009) (
Figure 2A). The incidence of more than moderate paravalvular leak was similar: 4.4% in the self-expanding valve-treated patients and 4.0% in the balloon-expandable valve-treated patients (
Figure 2B). These differences in hemodynamic outcomes between the 2 groups were maintained at 1-year follow-up. One-year follow-up echocardiography was available for 53 patients. At 1-year follow-up, the self-expanding valve-treated patients presented significantly larger EOA (1.67±0.32 vs. 1.27±0.44 cm
2, p<0.001) and iEOA (1.12±0.22 vs. 0.81±0.27 cm
2/m
2, p<0.001) compared with the balloon-expandable valve-treated patients (
Figure 1A and B). The incidence of PPM was less frequent in the self-expanding valve-treated patients (11.4% vs. 66.7% in balloon-expandable valve-treated patients, p<0.001) with no patients with severe PPM, while 33.3% of the balloon-expandable valve-treated patients presented severe PPM (
Figure 2A). The incidence of more than moderate paravalvular leak remained similar: 5.7% in the self-expanding valve-treated patients and 5.6% in the balloon-expandable valve-treated patients (
Figure 2B). The echocardiographic characteristics at baseline, discharge, and 1-year follow-up between the self-expanding 23 mm valve-treated patients and balloon-expandable 20 mm valve-treated patient are presented in
Supplementary Table 2.
Figure 1
Echocardiographic outcomes at baseline, discharge, and 1-year follow-up.
Effective orifice area (A), indexed effective orifice area (B), and mean aortic valve pressure gradient (C).
EOA = effective orifice area; iEOA = indexed effective orifice area.
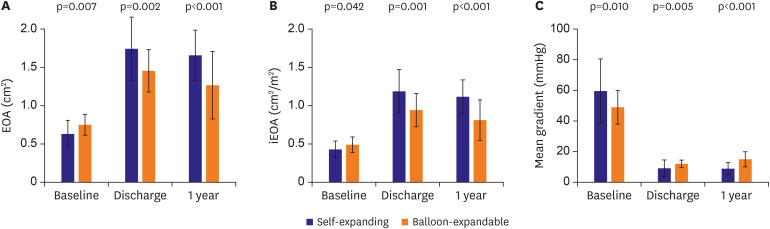

Figure 2
Incidence of prosthesis-patient mismatch and paravalvular leak at discharge and 1-year follow-up.
Prosthesis-patient mismatch (A) and paravalvular leak (B). There was no severe paravalvular leak during 1-year follow-up.
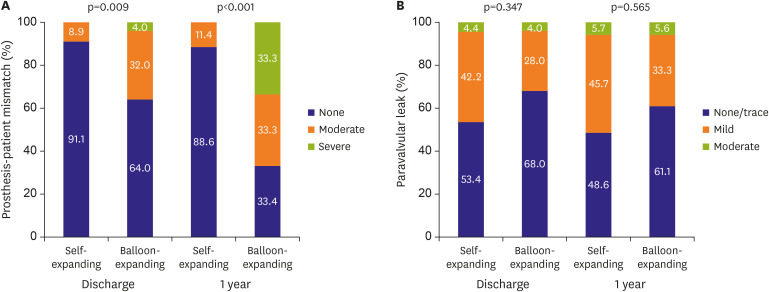

Table 4
Echocardiographic characteristics at discharge and 1-year follow-up
|
Overall (n=70) |
Self-expanding valve (n=45) |
Balloon-expandable valve (n=25) |
p value |
|
At discharge |
n=70 |
n=45 |
n=25 |
|
|
Effective orifice area (cm2) |
1.65±0.40 |
1.75±0.42 |
1.46±0.28 |
0.002 |
|
Indexed effective orifice area (cm2/m2) |
1.10±0.28 |
1.18±0.28 |
0.95±0.21 |
0.001 |
|
Maximum aortic valve pressure gradient (mmHg) |
19.7±9.0 |
17.5±9.7 |
23.6±5.7 |
0.001 |
|
Mean aortic valve pressure gradient (mmHg) |
9.9±4.7 |
8.9±5.2 |
11.7±2.9 |
0.005 |
|
Doppler velocity index |
0.61±0.15 |
0.66±0.14 |
0.51±0.10 |
<0.001 |
|
Prosthesis-patient mismatch |
13 (18.6) |
4 (8.9) |
9 (36.0) |
0.009 |
|
|
Moderate |
12 (17.1) |
4 (8.9) |
8 (32.0) |
|
|
Severe |
1 (1.4) |
0 (0.0) |
1 (4.0) |
|
Paravalvular leak |
29 (41.4) |
21 (46.7) |
8 (32.0) |
0.347 |
|
|
Mild |
26 (37.1) |
19 (42.2) |
7 (28.0) |
|
|
Moderate |
3 (4.3) |
2 (4.4) |
1 (4.0) |
|
|
Severe |
0 (0) |
0 (0) |
0 (0) |
|
Left ventricular ejection fraction (%) |
65.8±12.6 |
63.8±14.2 |
69.3±8.4 |
0.047 |
|
At 1-year follow-up*
|
n=53 |
n=35 |
n=18 |
|
|
Effective orifice area (cm2) |
1.53±0.41 |
1.67±0.32 |
1.27±0.44 |
<0.001 |
|
Indexed effective orifice area (cm2/m2) |
1.01±0.28 |
1.12±0.22 |
0.81±0.27 |
<0.001 |
|
Maximum aortic valve pressure gradient (mmHg) |
20.1±9.5 |
17.0±8.0 |
26.2±9.4 |
<0.001 |
|
Mean aortic valve pressure gradient (mmHg) |
10.6±5.2 |
8.5±4.0 |
14.7±4.8 |
<0.001 |
|
Doppler velocity index |
0.55±0.15 |
0.61±0.12 |
0.44±0.15 |
<0.001 |
|
Prosthesis-patient mismatch |
16 (30.2) |
4 (11.4) |
12 (66.7) |
<0.001 |
|
|
Moderate |
10 (18.9) |
4 (11.4) |
6 (33.3) |
|
|
Severe |
6 (11.3) |
0 (0.0) |
6 (33.3) |
|
Paravalvular leak |
25 (47.2) |
18 (51.4) |
7 (38.9) |
0.565 |
|
|
Mild |
22 (41.5) |
16 (45.7) |
6 (33.3) |
|
|
Moderate |
3 (5.7) |
2 (5.7) |
1 (5.6) |
|
|
Severe |
0 (0) |
0 (0) |
0 (0) |
|
Left ventricular ejection fraction (%) |
66.1±11.8 |
66.0±13.3 |
66.4±8.5 |
0.892 |

DISCUSSION
The main findings of this study are as follows: 1) Among Korean patients who underwent TAVR, a considerable percentage (25.8%) of patients presented with small aortic annulus. 2) TAVR in these patients was both effective and safe, and no significant difference in clinical outcomes was observed between the self-expanding and balloon-expandable valve-treated patients. 3) However, TAVR with the self-expanding valve was associated with more hemodynamic benefits with larger EOA, lower aortic valve pressure gradient, and consequently, lower incidence of PPM.
For current clinical practice, TAVR technology can be primarily classified into two types of valves: the self-expanding valve with supra-annular location or the balloon-expandable valve with intra-annular location. Although each type of valve has its own advantages and disadvantages, both types of valves have shown comparable clinical outcomes and superior hemodynamic outcomes compared with surgical aortic valve replacement; therefore, the use of TAVR has recently expanded.
1)2)3)4) Better hemodynamic benefits are driven from achievement of larger EOA in patients who received TAVR than in those who received surgical aortic valve replacement. Since the achievement of larger EOA depends on the original size of aortic annulus of each patient, the clinical benefit of TAVR toward valve hemodynamics was more pronounced in patients with small aortic annulus and selection for adequate TAVR valves is crucial to achieve larger EOA in these patients.
9)14) There have been studies comparing the performance of these two types of valves in small aortic annulus. Mauri et al. reported that compared with the balloon-expandable SAPIEN 3 valve, the self-expanding ACURATE neo valve showed superior hemodynamic outcomes (iEOA, 0.80 vs. 0.96 cm
2/m
2, p=0.003; mean aortic valve pressure gradient, 14.5 vs. 9.3 mmHg, p<0.001; severe PPM, 22% vs. 3%, p=0.004) in German patients with small aortic annulus defined as aortic annular area <400 mm
2 on computed tomography.
5) Rogers et al.
6) demonstrated similar results in patients in the United States with small aortic annulus defined as annular perimeter <73 mm on computed tomography. Compared with the balloon-expandable SAPIEN XT or SAPIEN 3 valve, the self-expanding CoreValve and Evoult R valves showed better hemodynamic outcomes (dimensionless index, 0.53 vs. 0.64, p=0.02; peak aortic valve velocity, 2.4 vs. 1.8 m/sec, p<0.001).
6) However, it remains uncertain which device performs better in Asian population with small aortic annulus. In our study, we specifically focused on Asian patients whose BSA was smaller than the Western population, adopted recently proposed definitions for small aortic annulus, and also compared the performance of the latest available generations of each type of valve with comparable size.
9) The improved hemodynamic outcomes were more prominent in patients who underwent TAVR with the self-expanding Evolut valve without significant differences in paravalvular leak and clinical outcomes, similar to previous studies in patients in Europe and the United States.
5)6) The main factor to achieve better hemodynamic benefits and larger EOA may be driven by supra-annular location in the self-expanding valve compared with intra-annular location in the balloon-expandable valve. Recently, Hase et al.
15) focused on Japanese patients with small aortic annulus defined as mean annulus diameter ≤23 mm on computed tomography. Compared with the balloon-expandable SAPIEN 3 valve, the self-expanding Evolut R valve showed superior hemodynamic outcomes (iEOA, 1.08 vs. 1.2 cm
2/m
2, p<0.001; mean aortic valve pressure gradient, 12.0 vs. 9.0 mmHg, p<0.001).
15) However, no significant difference was noted in the incidence of moderate and severe PPM which was different from our study. This may be due to the difference in study population with smaller BSA compared to the study of ours and Mauri et al.
5)
Clinically significant PPM was identified as a predictor of adverse clinical outcomes after TAVR.
2)9)16) According to the previous studies regarding patients with small aortic annulus who received the balloon-expandable SAPIEN valve, the incidence of more than moderate PPM was 39.4% in the Placement of Aortic Transcatheter Valves (PARTNER) trial and 42.1% in the study by Rogers et al.,
6) which were slightly higher than the 36.0% in our study.
14) On the other hand, the incidence of more than moderate PPM after self-expanding Evolut valve implantation in our study was considerably lower (8.9%) than a previous study which analyzed the performance of self-expanding valves in European patients with small aortic annulus (Evolut R, 24.5%; Evolut PRO, 20.5%).
17) The greater difference in the incidence of PPM following self-expanding valve implantation may be explained by several factors. Our definition of small aortic annulus was different from previous studies, as we followed the latest proposals for a mean diameter of <23 mm or minimal diameter of ≤21 mm on computed tomography.
9) Furthermore, our study specifically focused on the Asian population, whose BSA is relatively smaller than Western populations.
7)8) From the three randomized trials conducted in patients in the United States and Canada, Hahn et al.
18) reported the expected values of echocardiographic hemodynamic assessment after TAVR by valve type and size. The post-procedural EOA after 23 mm SAPIEN 3 valve implantation in our study was similar to the expected EOA (1.45±0.26 cm
2), however, the post-procedural EOA after 26 mm Evolut valve implantation was larger than the expected EOA (1.69±0.40 cm
2). Therefore, since PPM was defined according to iEOA which was calculated by dividing EOA by the BSA, the greater difference in the incidence of PPM following self-expanding Evolut valve implantation may be explained.
This study has several limitations. First, it was a single-center non-randomized study based on a limited number of patients with inherent limitations such as differences in the baseline clinical, procedural characteristics, and potential confounding factors. For instance, post-dilation which was more frequently performed the in the self-expanding valve-treated patients may have been an additional mechanism for the better hemodynamic outcomes apart from supra-annular location of the self-expanding valve. However, the impact of post-dilation cannot be fully evaluated in the present analysis. Second, we focused on patients with small aortic annulus and did not include other aortic annulus sizes, therefore patient selection bias may exist. Third, the choice of transcatheter aortic valve type was at the discretion of the operating interventional cardiologists. Fourth, 1-year echocardiography was not available in all patients.
In conclusion, in Korean patients with small aortic annulus, TAVR with self-expanding valves was associated with superior hemodynamic outcomes compared with balloon-expandable valves. These findings pave the way for further trials regarding appropriate prosthesis selection for TAVR in patients with small aortic annulus.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download