INTRODUCTION
The formation of herd immunity through vaccination is believed to be key to overcoming the coronavirus disease 2019 (COVID-19) pandemic. In Korea, vaccinations will begin in February 2021, with high-risk groups and COVID-19 medical staff being vaccinated first,
1 and by November 2021, the target is to reach a national population immunity level of 70%. Confidence in the safety of vaccines is a very important factor in national immunization.
Controversy over vaccine safety arose because of violation of the cold chain regulation during the transportation of influenza vaccination in Korea in 2020. Subsequently, competing media reports about deaths after influenza vaccination amplified public anxiety. Although it was found that there was no causal relationship, through autopsies for the deceased, comparisons with past death levels, and scientific investigations, it is difficult to overcome the loss of confidence in vaccines.
2
COVID-19 vaccines are raising concerns about their safety. Of particular concern, this is the first time the mRNA and viral vector platform has been used in large-scale immunization. There are case reports of anaphylaxis after vaccination with mRNA vaccine, and 11.1 cases of anaphylaxis per 1 million vaccinations in the United States (US) were reported in Pfizer's.
3 In clinical trials of AstraZeneca's vaccine, there have been reports of transverse myelitis, with controversy remaining over a causal relationship.
4
It is important to compare the natural incidence and the post-vaccination incidence, to determine the causal relationship between COVID-19 vaccination and conditions that may be potentially related to vaccine-related adverse events (VAEs). Natural incidence corresponds to the baseline incidence of each disease that occurs naturally in the absence of special interventions such as the COVID-19 vaccine. However, in Korea, the natural incidence of each condition is hardly measured.
In this study, we provide the basis for evaluating VAEs, verifying causality, and elucidating public concerns. We measured the baseline incidence rates of several conditions potentially related to VAEs through the National Health Insurance claim data. In addition, we aimed to present the expected incidence rates of those conditions for the year 2021 by analyzing the trends of their incidences over the past 15 years.
RESULTS
The incidence of vasovagal syncope has been relatively stable over the last decade, of which mean incidence was predicted to be 23.89 (95% CI, 19.8.1–27.98) per month. The incidence of anaphylaxis has been on a steady increase during last 15 years, and our model predicted that on average 4.72 (95% CI, 3.83–5.61) cases per 100,000 would occur a month in the year 2021 (
Fig. 1). However, a strong seasonality was noted (peak in summer) and seasonal variation was larger than secular change; thus, time of the year should be taken into consideration. Brachial neuritis has also been on the increase, but the degree of seasonality was smaller. The predicted mean monthly incidence in 2021 was 57.62 (95% CI, 51.37–63.88).
Fig. 1
Observed and predicted incidences of the study conditions. Green line denotes observed incidence from January 2006 through June 2020. Red line, fitted value; thick red line, predicted incidence from June 2020 through December 2021. Dark and light blue shade, 80% and 95% confidence intervals of prediction. Grey shade, the year 2021.
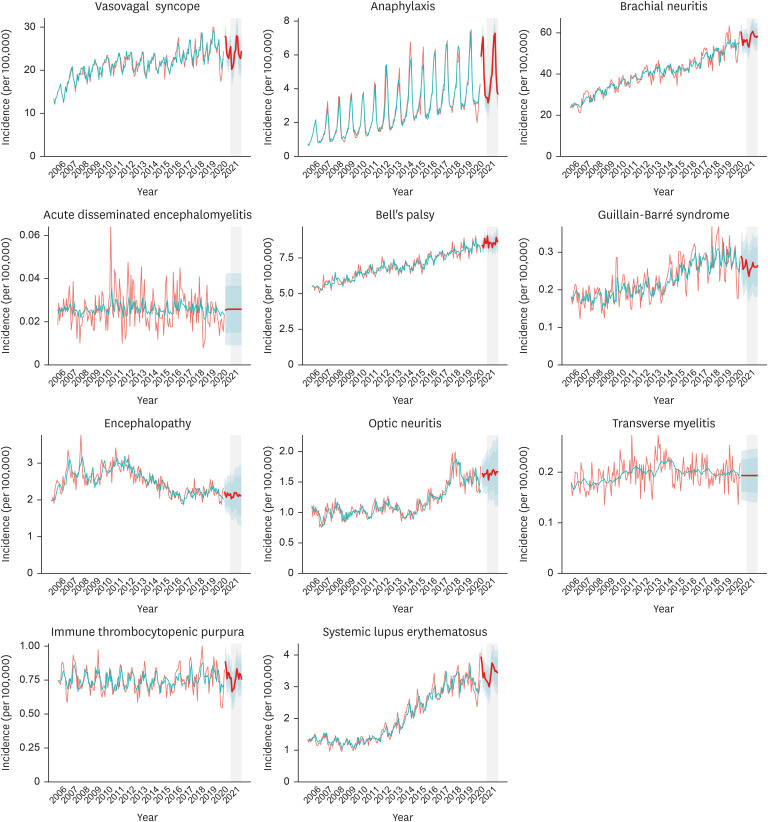

ADEM was extremely low, but its incidence has been constant. Encephalopathy (with no specific mention of etiology) has plateaued after declining in the early 2010s, while the incidence of optic neuritis became stable after increasing in the mid-2010s. The predicted mean monthly incidences were 2.13 (95% CI, 1.42–2.83) per month and 1.65 (95% CI, 1.17–2.13) per month, respectively. Bell's palsy and GBS have been on the increase through the last decade; our model predicted that 8.58 (95% CI, 7.90–9.26) cases of Bell's palsy and 0.26 (95% CI, 0.18–0.34) cases of GBS will occur every month in 2021. The incidence of ITP has been constant over time, of which predicted incidence was 0.75 (0.61–0.90) cases per month.
Subgroup analysis was performed on 4 age groups which are likely to be eligible for vaccination against COVID-19: 5–14 years, 15–24 years, 25–64 years, and ≥ 65 years (
Table 1 and
Supplementary Figs. 1-
4).
Table 1
Mean predicted monthly incidences of the conditions of interest
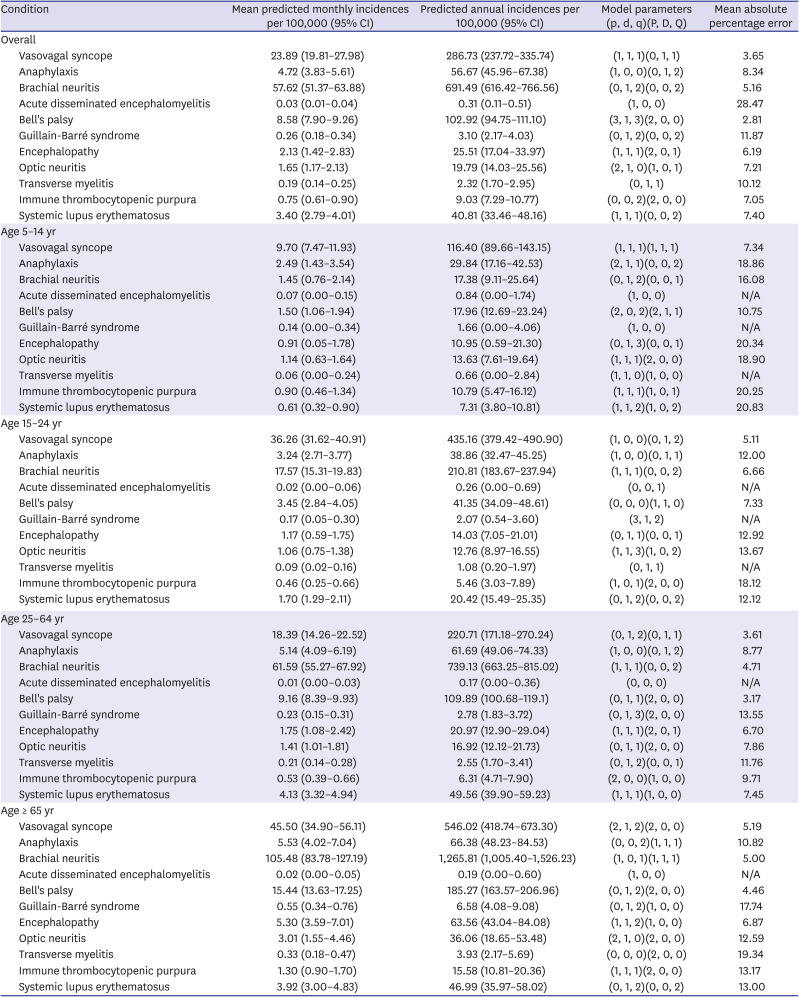
|
Condition |
Mean predicted monthly incidences per 100,000 (95% CI) |
Predicted annual incidences per 100,000 (95% CI) |
Model parameters (p, d, q)(P, D, Q) |
Mean absolute percentage error |
|
Overall |
|
|
|
|
|
Vasovagal syncope |
23.89 (19.81–27.98) |
286.73 (237.72–335.74) |
(1, 1, 1)(0, 1, 1) |
3.65 |
|
Anaphylaxis |
4.72 (3.83–5.61) |
56.67 (45.96–67.38) |
(1, 0, 0)(0, 1, 2) |
8.34 |
|
Brachial neuritis |
57.62 (51.37–63.88) |
691.49 (616.42–766.56) |
(0, 1, 2)(0, 0, 2) |
5.16 |
|
Acute disseminated encephalomyelitis |
0.03 (0.01–0.04) |
0.31 (0.11–0.51) |
(1, 0, 0) |
28.47 |
|
Bell's palsy |
8.58 (7.90–9.26) |
102.92 (94.75–111.10) |
(3, 1, 3)(2, 0, 0) |
2.81 |
|
Guillain-Barré syndrome |
0.26 (0.18–0.34) |
3.10 (2.17–4.03) |
(0, 1, 2)(0, 0, 2) |
11.87 |
|
Encephalopathy |
2.13 (1.42–2.83) |
25.51 (17.04–33.97) |
(1, 1, 1)(2, 0, 1) |
6.19 |
|
Optic neuritis |
1.65 (1.17–2.13) |
19.79 (14.03–25.56) |
(2, 1, 0)(1, 0, 1) |
7.21 |
|
Transverse myelitis |
0.19 (0.14–0.25) |
2.32 (1.70–2.95) |
(0, 1, 1) |
10.12 |
|
Immune thrombocytopenic purpura |
0.75 (0.61–0.90) |
9.03 (7.29–10.77) |
(0, 0, 2)(2, 0, 0) |
7.05 |
|
Systemic lupus erythematosus |
3.40 (2.79–4.01) |
40.81 (33.46–48.16) |
(1, 1, 1)(0, 0, 2) |
7.40 |
|
Age 5–14 yr |
|
|
|
|
|
Vasovagal syncope |
9.70 (7.47–11.93) |
116.40 (89.66–143.15) |
(1, 1, 1)(1, 1, 1) |
7.34 |
|
Anaphylaxis |
2.49 (1.43–3.54) |
29.84 (17.16–42.53) |
(2, 1, 1)(0, 0, 2) |
18.86 |
|
Brachial neuritis |
1.45 (0.76–2.14) |
17.38 (9.11–25.64) |
(0, 1, 2)(0, 0, 1) |
16.08 |
|
Acute disseminated encephalomyelitis |
0.07 (0.00–0.15) |
0.84 (0.00–1.74) |
(1, 0, 0) |
N/A |
|
Bell's palsy |
1.50 (1.06–1.94) |
17.96 (12.69–23.24) |
(2, 0, 2)(2, 1, 1) |
10.75 |
|
Guillain-Barré syndrome |
0.14 (0.00–0.34) |
1.66 (0.00–4.06) |
(1, 0, 0) |
N/A |
|
Encephalopathy |
0.91 (0.05–1.78) |
10.95 (0.59–21.30) |
(0, 1, 3)(0, 0, 1) |
20.34 |
|
Optic neuritis |
1.14 (0.63–1.64) |
13.63 (7.61–19.64) |
(1, 1, 1)(2, 0, 0) |
18.90 |
|
Transverse myelitis |
0.06 (0.00–0.24) |
0.66 (0.00–2.84) |
(1, 1, 0)(1, 0, 0) |
N/A |
|
Immune thrombocytopenic purpura |
0.90 (0.46–1.34) |
10.79 (5.47–16.12) |
(1, 1, 1)(1, 0, 1) |
20.25 |
|
Systemic lupus erythematosus |
0.61 (0.32–0.90) |
7.31 (3.80–10.81) |
(1, 1, 2)(1, 0, 2) |
20.83 |
|
Age 15–24 yr |
|
|
|
|
|
Vasovagal syncope |
36.26 (31.62–40.91) |
435.16 (379.42–490.90) |
(1, 0, 0)(0, 1, 2) |
5.11 |
|
Anaphylaxis |
3.24 (2.71–3.77) |
38.86 (32.47–45.25) |
(1, 0, 0)(0, 1, 1) |
12.00 |
|
Brachial neuritis |
17.57 (15.31–19.83) |
210.81 (183.67–237.94) |
(1, 1, 1)(0, 0, 2) |
6.66 |
|
Acute disseminated encephalomyelitis |
0.02 (0.00–0.06) |
0.26 (0.00–0.69) |
(0, 0, 1) |
N/A |
|
Bell's palsy |
3.45 (2.84–4.05) |
41.35 (34.09–48.61) |
(0, 0, 0)(1, 1, 0) |
7.33 |
|
Guillain-Barré syndrome |
0.17 (0.05–0.30) |
2.07 (0.54–3.60) |
(3, 1, 2) |
N/A |
|
Encephalopathy |
1.17 (0.59–1.75) |
14.03 (7.05–21.01) |
(0, 1, 1)(0, 0, 1) |
12.92 |
|
Optic neuritis |
1.06 (0.75–1.38) |
12.76 (8.97–16.55) |
(1, 1, 3)(1, 0, 2) |
13.67 |
|
Transverse myelitis |
0.09 (0.02–0.16) |
1.08 (0.20–1.97) |
(0, 1, 1) |
N/A |
|
Immune thrombocytopenic purpura |
0.46 (0.25–0.66) |
5.46 (3.03–7.89) |
(1, 0, 1)(2, 0, 0) |
18.12 |
|
Systemic lupus erythematosus |
1.70 (1.29–2.11) |
20.42 (15.49–25.35) |
(0, 1, 2)(0, 0, 2) |
12.12 |
|
Age 25–64 yr |
|
|
|
|
|
Vasovagal syncope |
18.39 (14.26–22.52) |
220.71 (171.18–270.24) |
(0, 1, 2)(0, 1, 1) |
3.61 |
|
Anaphylaxis |
5.14 (4.09–6.19) |
61.69 (49.06–74.33) |
(1, 0, 0)(0, 1, 2) |
8.77 |
|
Brachial neuritis |
61.59 (55.27–67.92) |
739.13 (663.25–815.02) |
(1, 1, 1)(0, 0, 2) |
4.71 |
|
Acute disseminated encephalomyelitis |
0.01 (0.00–0.03) |
0.17 (0.00–0.36) |
(0, 0, 0) |
N/A |
|
Bell's palsy |
9.16 (8.39–9.93) |
109.89 (100.68–119.1) |
(0, 1, 1)(2, 0, 0) |
3.17 |
|
Guillain-Barré syndrome |
0.23 (0.15–0.31) |
2.78 (1.83–3.72) |
(0, 1, 3)(2, 0, 0) |
13.55 |
|
Encephalopathy |
1.75 (1.08–2.42) |
20.97 (12.90–29.04) |
(1, 1, 1)(2, 0, 1) |
6.70 |
|
Optic neuritis |
1.41 (1.01–1.81) |
16.92 (12.12–21.73) |
(0, 1, 1)(2, 0, 0) |
7.86 |
|
Transverse myelitis |
0.21 (0.14–0.28) |
2.55 (1.70–3.41) |
(0, 1, 2)(0, 0, 1) |
11.76 |
|
Immune thrombocytopenic purpura |
0.53 (0.39–0.66) |
6.31 (4.71–7.90) |
(2, 0, 0)(1, 0, 0) |
9.71 |
|
Systemic lupus erythematosus |
4.13 (3.32–4.94) |
49.56 (39.90–59.23) |
(1, 1, 1)(1, 0, 0) |
7.45 |
|
Age ≥ 65 yr |
|
|
|
|
|
Vasovagal syncope |
45.50 (34.90–56.11) |
546.02 (418.74–673.30) |
(2, 1, 2)(2, 0, 0) |
5.19 |
|
Anaphylaxis |
5.53 (4.02–7.04) |
66.38 (48.23–84.53) |
(0, 0, 2)(1, 1, 1) |
10.82 |
|
Brachial neuritis |
105.48 (83.78–127.19) |
1,265.81 (1,005.40–1,526.23) |
(1, 0, 1)(1, 1, 1) |
5.00 |
|
Acute disseminated encephalomyelitis |
0.02 (0.00–0.05) |
0.19 (0.00–0.60) |
(1, 0, 0) |
N/A |
|
Bell's palsy |
15.44 (13.63–17.25) |
185.27 (163.57–206.96) |
(0, 1, 2)(2, 0, 0) |
4.46 |
|
Guillain-Barré syndrome |
0.55 (0.34–0.76) |
6.58 (4.08–9.08) |
(0, 1, 2)(1, 0, 0) |
17.74 |
|
Encephalopathy |
5.30 (3.59–7.01) |
63.56 (43.04–84.08) |
(1, 1, 2)(1, 0, 0) |
6.87 |
|
Optic neuritis |
3.01 (1.55–4.46) |
36.06 (18.65–53.48) |
(2, 1, 0)(2, 0, 0) |
12.59 |
|
Transverse myelitis |
0.33 (0.18–0.47) |
3.93 (2.17–5.69) |
(0, 0, 0)(2, 0, 0) |
19.34 |
|
Immune thrombocytopenic purpura |
1.30 (0.90–1.70) |
15.58 (10.81–20.36) |
(1, 1, 1)(2, 0, 0) |
13.17 |
|
Systemic lupus erythematosus |
3.92 (3.00–4.83) |
46.99 (35.97–58.02) |
(0, 1, 2)(0, 0, 2) |
13.00 |

DISCUSSION
We measured and followed the trajectories of the incidence of a total of 11 conditions that could potentially be associated with VAEs. Based on these analyses, we predicted the monthly incidence of the conditions for the year 2021. Seasonality was observed in the majority of conditions, especially anaphylaxis, which showed strong seasonality. However, it was difficult to observe seasonality for conditions with low incidence, such as ADEM and transverse myelitis.
The incidence of the majority of conditions tended to increase. Vasovagal syncope, anaphylaxis, brachial neuritis, Bell's palsy, GBS, and SLE continued to increase during the observation period. This can be explained by improved reporting from medical staff, advances in diagnosis technology for diseases, enhancement of public awareness, and changing claims patterns, but additional research is needed on specific causes for each disease.
Vasovagal syncope and anaphylaxis have been found in 23.89 and 4.72 new cases per 100,000 people per month, and it is possible that cases could develop at COVID-19 vaccination sites, so thorough preparations are required. There were few previous studies on the epidemiology of vasovagal syncope in Korea. Anaphylaxis continued to increase in previous studies conducted in Korea, which is consistent with our findings.
9 In particular, since anaphylaxis is mainly caused by external factors, it is more important to use the real-world report in the country in which the vaccination is actually administered rather than the baseline incidence, which is influenced by many external factors. In the US, a total of 17 million doses of mRNA vaccination had been administered as of January 18, 2021, and the incidence of anaphylaxis has been reported to be 4.7 cases/million doses for the Pfizer vaccine and 2.5 cases/million doses for the Moderna vaccine.
10 In the United Kingdom (UK), 6.6 million doses of the Pfizer vaccine and 3 million doses of the AstraZeneca vaccine had been administered as of January 31, 2021, and the incidence of anaphylaxis for the Pfizer vaccine was reported to be 10 to 20 cases per million doses, higher than that in the US.
11
Similarly, in ADEM and GBS, epidemiology in children was studied in Korea between 2010 and 2017,
12 and the pattern of increase was found. The incidence rate of GBS related to the 2009–2010 influenza pandemic was reported to be 0.87 cases per 100,000 person-years during the pandemic period, and 0.63 cases in the baseline period.
13 The annual incidence of optic neuritis per 100,000 people in Korea between and 2010–2016 was reported to be 16.36.
14 Prior studies of primary ITP reported an incidence of 5.3 per 100,000 person-years by 2010–2014
15, also showing similar patterns. SLE was suggested to have 35.45 prevalent cases per 100,000 person-years in previous studies, with a reduction reported in the actual incidence.
16 This is the opposite trend to the increase observed in our study. This may be because our study prioritized the detection of disease occurrence rather than a strict operational definition. For brachial neuritis, Bell's palsy, encephalopathy, and optic neuritis, we could not find adequate prior studies.
The US manages the VAE through the Vaccine Safety Datalink. Surveillance of multiple VAEs, such as anaphylaxis and GBS, is regularly performed.
1718 The lack of such systematic surveillance in Korea is a pitfall at a time of large-scale vaccination. In addition, countries that are vaccinating such as the US and the UK are operating VAE monitoring programs that actively utilize the Internet and mobile apps called V-safe
19 and Yellow card schemes,
20 and disclose information obtained through them in near real time. These measures are of great help in resolving the public's mistrust of vaccines. Korea also needs to introduce such a systematic surveillance system.
This study presents national trends that could potentially be related to VAEs using health insurance claims data. Therefore, it has the inherent limitations of research using claims data. The main problem is the accuracy of the claims. To detect the condition of interest, this study used an operational definition based on only the ICD-10 code. Diagnostic codes claimed by clinics in charge of primary care may be inaccurate, and in particular, the diseases covered in this study are very difficult to diagnose and the problem may be more pronounced. To address this problem, there are methods of including drugs and treatment material prescriptions in health insurance claims data in the operational definition, or including only claims through medical record review such as “co-payment on the rare incurable diseases codes.” However, our study did not use this method for comprehensive and reproducible measurements of various diseases. Also, the co-payment policy on the rare incurable diseases has been implemented since 2009 for limited diseases, and does not include all of our target diseases and observation period. In addition, it was considered that it would be practical to use as broad a definition as possible, as a comprehensive report would be made on the side effects of concern after vaccination. Also there is a possibility of overestimation. In addition to the aforementioned diagnostic code problem, this study applied a 3-year washout period. Therefore, the incidence of diseases with frequent recurrence and poor treatment adherence may be overestimated.
Another interpretation caveat is that the baseline incidence of conditions that may be related to the VAEs reported through this study is not the value measured immediately after a specific vaccination. This baseline is measured in a natural state, and reflects all the circumstances of the past 15 years, such as seasonal flu vaccination and the H1N1 influenza pandemic, and appears as a monthly average for 15 years. In particular, GBS and anaphylaxis appeared to increase rapidly in 2009 in certain age groups, which may be related to the H1N1 influenza pandemic in 2009-10.
The CI level of this study's prediction was presented as 95%, but this was not adjusted in terms of multiple testing comparing 11 conditions and 4 age-specific sub-groups. However, the method for obtaining the multiplicity-corrected CIs for future predictions is not clearly established. Therefore, additional research is needed to determine the optimal significance level in prediction using a time series model.
In order to utilize the results of this study, it is necessary to collect thorough cases of suspicious VAEs. In addition, since the causal relationship with vaccination has not been established for other conditions except anaphylaxis, caution in interpretation is required. For example, if cases of transverse myelitis are reported following COVID-19 vaccination, these cases should be divided into administered doses and the observation period. When the incidence exceeds our monthly average incidence, it could be considered an adverse event from the vaccine. On the other hand, if the observed incidence rate is within the expected range, this suggests that this phenomenon is due to natural chance rather than caused by the vaccine.
In conclusion, in Korea, conditions that could potentially be related to VAEs occur on a regular basis, and an increase in the majority of the conditions is observed with seasonality. In addition, improved diagnostic technology, reporting, and medical utilization for rare diseases mean that there may be a higher level of disease reporting than in the past. Our results predict the incidence of VAEs in advance of national COVID-19 vaccination in Korea.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download