1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010; 363(17):1597–1607. PMID:
20961243.

2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019; 380(18):1695–1705. PMID:
30883058.


3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019; 380(18):1706–1715. PMID:
30883053.


4. Sohn SH, Jang MJ, Hwang HY, Kim KH. Rapid deployment or sutureless versus conventional bioprosthetic aortic valve replacement: a meta-analysis. J Thorac Cardiovasc Surg. 2018; 155(6):2402–2412.e5. PMID:
29548584.


5. Wahlers TC, Haverich A, Borger MA, Shrestha M, Kocher AA, Walther T, et al. Early outcomes after isolated aortic valve replacement with rapid deployment aortic valve. J Thorac Cardiovasc Surg. 2016; 151(6):1639–1647. PMID:
26892076.


6. Rahmanian PB, Kaya S, Eghbalzadeh K, Menghesha H, Madershahian N, Wahlers T. Rapid deployment aortic valve replacement: excellent results and increased effective orifice area. Ann Thorac Surg. 2018; 105(1):24–30. PMID:
29132703.

7. Ranucci M, Frigiola A, Menicanti L, Castelvecchio S, de Vincentiis C, Pistuddi V. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis. 2012; 21(6):732–739. PMID:
23409353.

8. Al-Sarraf N, Thalib L, Hughes A, Houlihan M, Tolan M, Young V, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg. 2011; 9(1):104–109. PMID:
20965288.


9. Iino K, Miyata H, Motomura N, Watanabe G, Tomita S, Takemura H, et al. Prolonged cross-clamping during aortic valve replacement is an independent predictor of postoperative morbidity and mortality: analysis of the Japan cardiovascular surgery database. Ann Thorac Surg. 2017; 103(2):602–609. PMID:
27624296.


10. Ensminger S, Fujita B, Bauer T, Möllmann H, Beckmann A, Bekeredjian R, et al. Rapid deployment versus conventional bioprosthetic valve replacement for aortic stenosis. J Am Coll Cardiol. 2018; 71(13):1417–1428. PMID:
29598861.


11. Moon TJ. Light and shadows of the Korean healthcare system. J Korean Med Sci. 2012; 27(Suppl):S3–S6. PMID:
22661868.

12. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014; 38(5):395–403. PMID:
25349827.



13. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004; 57(12):1288–1294. PMID:
15617955.


14. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017; 36(27):4391–4400. PMID:
28913837.



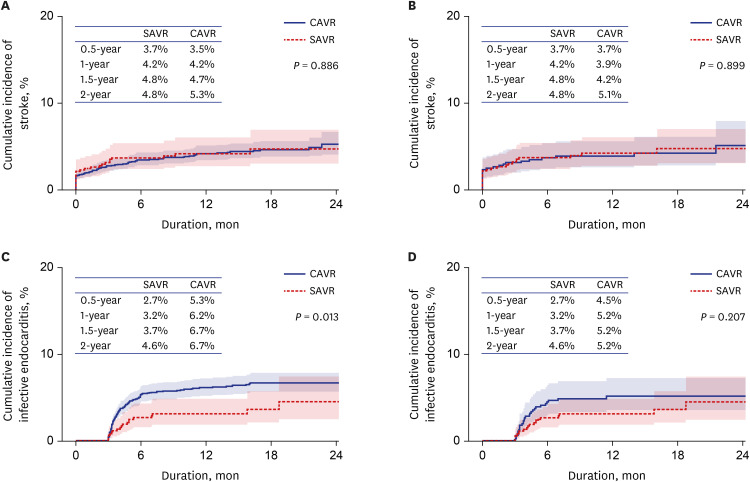
15. Barnhart GR, Accola KD, Grossi EA, Woo YJ, Mumtaz MA, Sabik JF, et al. TRANSFORM (multicenter experience with rapid deployment EDWARDS INTUITY valve system for aortic valve replacement) US clinical trial: performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg. 2017; 153(2):241–251.e2. PMID:
27817951.


16. Laufer G, Haverich A, Andreas M, Mohr FW, Walther T, Shrestha M, et al. Long-term outcomes of a rapid deployment aortic valve: data up to 5 years. Eur J Cardiothorac Surg. 2017; 52(2):281–287. PMID:
28453629.


17. Young C, Laufer G, Kocher A, Solinas M, Alamanni F, Polvani G, et al. One-year outcomes after rapid-deployment aortic valve replacement. J Thorac Cardiovasc Surg. 2018; 155(2):575–585. PMID:
29415382.


18. Fischlein T, Meuris B, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, et al. The sutureless aortic valve at 1 year: a large multicenter cohort study. J Thorac Cardiovasc Surg. 2016; 151(6):1617–1626.e4. PMID:
26936009.

19. Shrestha M, Fischlein T, Meuris B, Flameng W, Carrel T, Madonna F, et al. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg. 2016; 49(1):234–241. PMID:
25750010.


20. Di Eusanio M, Phan K, Berretta P, Carrel TP, Andreas M, Santarpino G, et al. Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR): early results from 3343 patients. Eur J Cardiothorac Surg. 2018; 54(4):768–773. PMID:
29617925.


21. Berretta P, Andreas M, Carrel TP, Solinas M, Teoh K, Fischlein T, et al. Minimally invasive aortic valve replacement with sutureless and rapid deployment valves: a report from an international registry (Sutureless and Rapid Deployment International Registry). Eur J Cardiothorac Surg. 2019; 56(4):793–799. PMID:
30820549.


22. Gilmanov D, Miceli A, Ferrarini M, Farneti P, Murzi M, Solinas M, et al. Aortic valve replacement through right anterior minithoracotomy: can sutureless technology improve clinical outcomes? Ann Thorac Surg. 2014; 98(5):1585–1592. PMID:
25200732.


23. Muneretto C, Alfieri O, Cesana BM, Bisleri G, De Bonis M, Di Bartolomeo R, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real-world” patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg. 2015; 150(6):1570–1577. PMID:
26384753.


24. Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S, et al. Sutureless Perceval aortic valve versus conventional stented bioprostheses: Meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc. 2018; 7(4):e006091. PMID:
29453309.

25. Andreas M, Wallner S, Habertheuer A, Rath C, Schauperl M, Binder T, et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact Cardiovasc Thorac Surg. 2016; 22(6):799–805. PMID:
26976130.



26. Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg. 2013; 145(1):110–115. PMID:
23058665.

27. Flameng W, Herregods MC, Hermans H, Van der Mieren G, Vercalsteren M, Poortmans G, et al. Effect of sutureless implantation of the Perceval S aortic valve bioprosthesis on intraoperative and early postoperative outcomes. J Thorac Cardiovasc Surg. 2011; 142(6):1453–1457. PMID:
21474151.


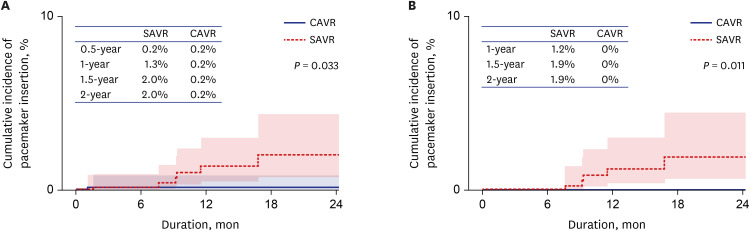
28. Mehaffey JH, Haywood NS, Hawkins RB, Kern JA, Teman NR, Kron IL, et al. Need for permanent pacemaker after surgical aortic valve replacement reduces long-term survival. Ann Thorac Surg. 2018; 106(2):460–465. PMID:
29577930.



29. Greason KL, Lahr BD, Stulak JM, Cha YM, Rea RF, Schaff HV, et al. Long-term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg. 2017; 104(4):1259–1264. PMID:
28433222.


30. Concistrè G, Chiaramonti F, Bianchi G, Cerillo A, Murzi M, Margaryan R, et al. Aortic valve replacement with Perceval bioprosthesis: single-center experience with 617 implants. Ann Thorac Surg. 2018; 105(1):40–46. PMID:
28964415.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download