1. Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015; 16(2):93–98. PMID:
25577163.



2. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989; 69(3):990–1047. PMID:
2664828.


3. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130(3):456–469. PMID:
17693256.



4. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012; 50(2):568–575. PMID:
21550430.


5. Liu JM, Rosen CJ, Ducy P, Kousteni S, Karsenty G. Regulation of glucose handling by the skeleton: insights from mouse and human studies. Diabetes. 2016; 65(11):3225–3232. PMID:
27959858.



6. Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014; 63(3):1021–1031. PMID:
24009262.



7. Sabek OM, Nishimoto SK, Fraga D, Tejpal N, Ricordi C, Gaber AO. Osteocalcin effect on human β-cells mass and function. Endocrinology. 2015; 156(9):3137–3146. PMID:
26151356.


8. Funakoshi S, Yoshimura K, Hirano S, Ohmi S, Amano E, Fukuda Y, et al. Undercarboxylated osteocalcin correlates with insulin secretion in Japanese individuals with diabetes. Diabetol Metab Syndr. 2020; 12(1):72. PMID:
32821293.



9. Díaz-López A, Bulló M, Juanola-Falgarona M, Martínez-González MA, Estruch R, Covas MI, et al. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metab. 2013; 98(11):4524–4531. PMID:
24037881.


10. Ngarmukos C, Chailurkit LO, Chanprasertyothin S, Hengprasith B, Sritara P, Ongphiphadhanakul B. A reduced serum level of total osteocalcin in men predicts the development of diabetes in a long-term follow-up cohort. Clin Endocrinol (Oxf). 2012; 77(1):42–46. PMID:
21916911.


11. Liu DM, Guo XZ, Tong HJ, Tao B, Sun LH, Zhao HY, et al. Association between osteocalcin and glucose metabolism: a meta-analysis. Osteoporos Int. 2015; 26(12):2823–2833. PMID:
26089135.


12. Riquelme-Gallego B, García-Molina L, Cano-Ibáñez N, Sánchez-Delgado G, Andújar-Vera F, García-Fontana C, et al. Circulating undercarboxylated osteocalcin as estimator of cardiovascular and type 2 diabetes risk in metabolic syndrome patients. Sci Rep. 2020; 10(1):1840. PMID:
32020009.



13. Shu H, Pei Y, Chen K, Lu J. Significant inverse association between serum osteocalcin and incident type 2 diabetes in a middle-aged cohort. Diabetes Metab Res Rev. 2016; 32(8):867–874. PMID:
27061949.


14. Urano T, Shiraki M, Kuroda T, Tanaka S, Urano F, Uenishi K, et al. Low serum osteocalcin concentration is associated with incident type 2 diabetes mellitus in Japanese women. J Bone Miner Metab. 2018; 36(4):470–477. PMID:
28766135.


15. Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015; 30(8):599–614. PMID:
26085114.


16. Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009; 161(5):723–729. PMID:
19671707.


17. Bae SJ, Choe JW, Chung YE, Kim BJ, Lee SH, Kim HY, et al. The association between serum osteocalcin levels and metabolic syndrome in Koreans. Osteoporos Int. 2011; 22(11):2837–2846. PMID:
21153019.


18. Yang R, Ma X, Pan X, Wang F, Luo Y, Gu C, et al. Serum osteocalcin levels in relation to metabolic syndrome in Chinese postmenopausal women. Menopause. 2013; 20(5):548–553. PMID:
23615646.


19. Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008; 396(1-2):66–69. PMID:
18657532.

20. Choudhury AB, Sarkar PD, Sakalley DK, Petkar SB. Role of adiponectin in mediating the association of osteocalcin with insulin resistance and type 2 diabetes: a cross sectional study in pre- and post-menopausal women. Arch Physiol Biochem. 2014; 120(2):73–79. PMID:
24405382.

21. Kim S, Lee JY, Im JA, Kim DW, Lee HS, Kim SH, et al. Association between serum osteocalcin and insulin resistance in postmenopausal, but not premenopausal, women in Korea. Menopause. 2013; 20(10):1061–1066. PMID:
23632656.


22. Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, et al. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism. 2011; 60(8):1186–1192. PMID:
21353261.


23. Lee SW, Jo HH, Kim MR, Kim JH, You YO. Association between osteocalcin and metabolic syndrome in postmenopausal women. Arch Gynecol Obstet. 2015; 292(3):673–681. PMID:
25716667.

24. Gundberg CM, Looker AC, Nieman SD, Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002; 31(6):703–708. PMID:
12531565.


25. Yasui T, Uemura H, Tomita J, Miyatani Y, Yamada M, Miura M, et al. Association of serum undercarboxylated osteocalcin with serum estradiol in pre-, peri- and early post-menopausal women. J Endocrinol Invest. 2006; 29(10):913–918. PMID:
17185901.


26. Kim CJ, Kim TH, Ryu WS, Ryoo UH. Influence of menopause on high density lipoprotein-cholesterol and lipids. J Korean Med Sci. 2000; 15(4):380–386. PMID:
10983684.



27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28(7):412–419. PMID:
3899825.


28. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23(5):469–480. PMID:
16681555.


29. Hong AR, Lim S. Clinical characteristics of metabolic syndrome in Korea, and its comparison with other Asian countries. J Diabetes Investig. 2015; 6(5):508–515.


30. Park SH, Yoon JS, Won KC, Lee HW. Usefulness of glycated hemoglobin as diagnostic criteria for metabolic syndrome. J Korean Med Sci. 2012; 27(9):1057–1061. PMID:
22969252.



31. Vanderschueren D, Gevers G, Raymaekers G, Devos P, Dequeker J. Sex- and age-related changes in bone and serum osteocalcin. Calcif Tissue Int. 1990; 46(3):179–182. PMID:
2106376.


32. Atalay S, Elci A, Kayadibi H, Onder CB, Aka N. Diagnostic utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann Lab Med. 2012; 32(1):23–30. PMID:
22259775.


33. Park SG, Jeong SU, Lee JH, Ryu SH, Jeong HJ, Sim YJ, et al. The changes of CTX, DPD, osteocalcin, and bone mineral density during the postmenopausal period. Ann Rehabil Med. 2018; 42(3):441–448. PMID:
29961742.



34. Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001; 4(4):267–272. PMID:
11770182.


35. Park CY, Lim JY, Park HY. Age at natural menopause in Koreans: secular trends and influences thereon. Menopause. 2018; 25(4):423–429. PMID:
29112598.


36. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010; 142(2):309–319. PMID:
20655471.



37. Desentis-Desentis MF, Rivas-Carrillo JD, Sánchez-Enríquez S. Protective role of osteocalcin in diabetes pathogenesis. J Bone Miner Metab. 2020; 38(6):765–771. PMID:
32725267.


38. Mori K, Emoto M, Motoyama K, Lee E, Yamada S, Morioka T, et al. Undercarboxylated osteocalcin does not correlate with insulin resistance as assessed by euglycemic hyperinsulinemic clamp technique in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012; 4(1):53. PMID:
23249601.



39. D'Amelio P, Sassi F, Buondonno I, Spertino E, Tamone C, Piano S, et al. Effect of intermittent PTH treatment on plasma glucose in osteoporosis: a randomized trial. Bone. 2015; 76:177–184. PMID:
25827255.

40. Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. 2016; 34(4):367–379. PMID:
26747614.


41. Jørgensen CV, Gasparini SJ, Tu J, Zhou H, Seibel MJ, Bräuner-Osborne H. Metabolic and skeletal homeostasis are maintained in full locus GPRC6A knockout mice. Sci Rep. 2019; 9(1):5995. PMID:
30979912.



42. Sanchez-Enriquez S, Ballesteros-Gonzalez IT, Villafán-Bernal JR, Pascoe-Gonzalez S, Rivera-Leon EA, Bastidas-Ramirez BE, et al. Serum levels of undercarboxylated osteocalcin are related to cardiovascular risk factors in patients with type 2 diabetes mellitus and healthy subjects. World J Diabetes. 2017; 8(1):11–17. PMID:
28138360.



43. Xu Y, Ma X, Xiong Q, Hu X, Zhang X, Yuan Y, et al. Association between serum osteocalcin level and blood pressure in a Chinese population. Blood Press. 2018; 27(2):106–111. PMID:
29172726.


44. Chen L, Li Q, Yang Z, Ye Z, Huang Y, He M, et al. Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese. Diabet Med. 2013; 30(3):309–317. PMID:
22913521.


45. Oosterwerff MM, van Schoor NM, Lips P, Eekhoff EM. Osteocalcin as a predictor of the metabolic syndrome in older persons: a population-based study. Clin Endocrinol (Oxf). 2013; 78(2):242–247. PMID:
22435398.


46. Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002; 51(10):3120–3127. PMID:
12351457.


47. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002; 288(21):2709–2716. PMID:
12460094.

48. Millar SA, Patel H, Anderson SI, England TJ, O'Sullivan SE. Osteocalcin, vascular calcification, and atherosclerosis: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2017; 8:183. PMID:
28824544.



49. Zhou S, Fang X, Xin H, Li W, Qiu H, Guan S. Osteoprotegerin inhibits calcification of vascular smooth muscle cell via down regulation of the Notch1-RBP-Jκ/Msx2 signaling pathway. PLoS One. 2013; 8(7):e68987. PMID:
23874840.

50. Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011; 31(9):e55–71. PMID:
21757657.

51. Pal SN, Rush C, Parr A, Van Campenhout A, Golledge J. Osteocalcin positive mononuclear cells are associated with the severity of aortic calcification. Atherosclerosis. 2010; 210(1):88–93. PMID:
20004897.


52. Akiyoshi T, Ota H, Iijima K, Son BK, Kahyo T, Setou M, et al. A novel organ culture model of aorta for vascular calcification. Atherosclerosis. 2016; 244:51–58. PMID:
26584139.


53. Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018; 10(7):847.


54. Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun. 2010; 397(4):691–696. PMID:
20570657.

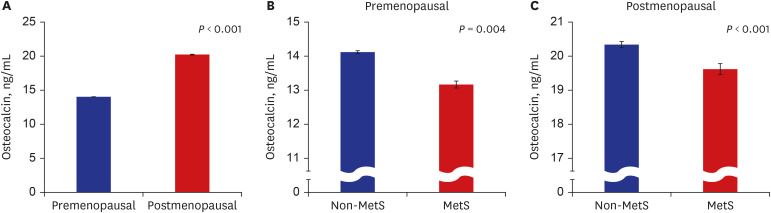
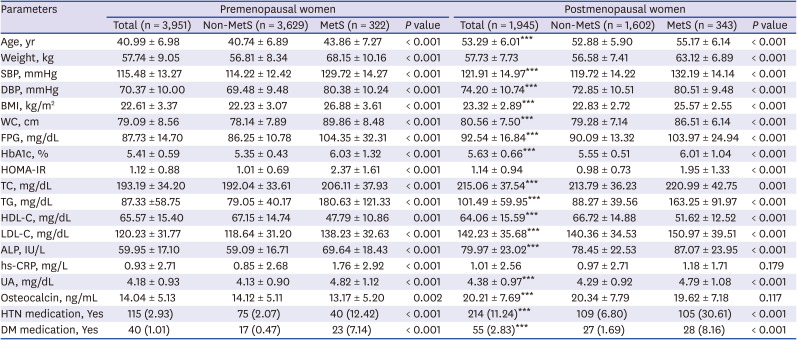
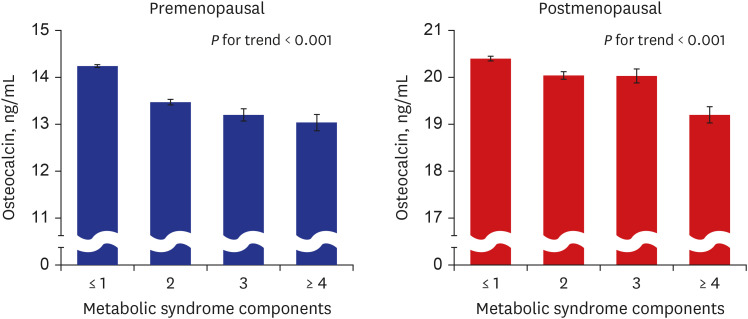
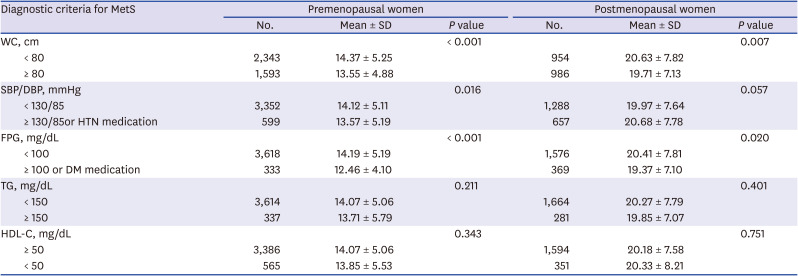




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download