INTRODUCTION
Dendritic cell is a cell that participates in immune response activity as an antigen-presenting cell by stimulation of T lymphocytes. Among them, interdigitating dendritic cell sarcoma (IDCS) is an extremely rare malignant hematopoietic tumor derived from interdigitating dendritic cells, with fewer than 200 cases reported [
1].
Although most of IDCS arise in lymph nodes, IDCS occurs at extranodal sites, such as the lung, skin, breast, bone, liver, spleen, small intestine, nasopharynx, urinary bladder, and head and neck area [
2-
5].
Herein, we report a case of a woman who had a tenderness, rapidly growing ulcerative lesion on her skin of the upper arm. It was first misdiagnosed as a ruptured epidermal inclusion cyst according to clinical features and ultrasound findings, however, subsequently diagnosed as IDCS by postoperative histopathology.
Go to :

CASE REPORT
A 66-year-old woman visited a local clinic 5 months ago with a mass on her right upper arm. According to her, the mass started from small bullae about 1 year earlier and then enlarged continually. She visited a local clinician, and the presumptive diagnosis was dermatitis and triamcinolone acetonide injections were prescribed. However, the medication did not work and the lesion kept worsening, then she was referred to our outpatient dermatology clinic. On physical examination of her right upper arm, about 3×3-cm sized mass with mild tenderness was located in subcutaneous layer (
Fig. 1A). The dermatologist’s impression was a benign neoplasm with inflammation or keloid formation; therefore, the patient was prescribed with oral antibiotics (first-generation cephalosporin) and ultrasonography.
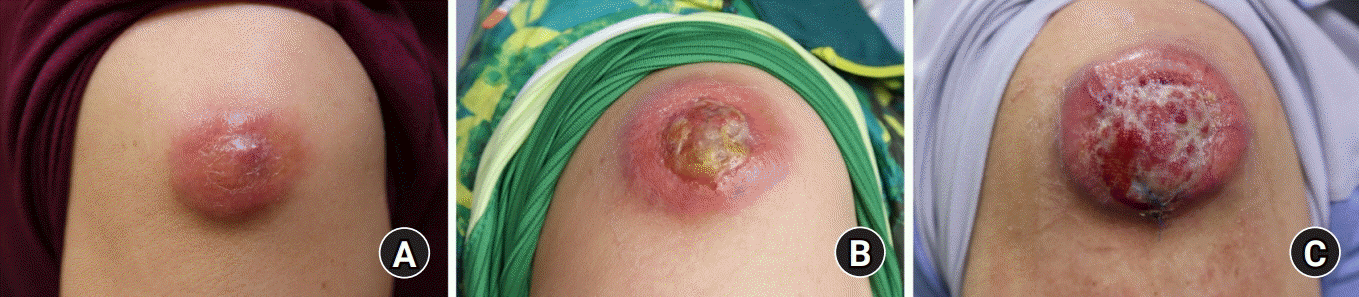 | Fig. 1.The tumor enlarged in size during the patient’s following visits. (A) Approximately 3×3-cm sized mass with mild tenderness was located in the subcutaneous layer at the beginning. (B) After 2 months, the patient was referred to the plastic surgery department for more protruding with erythematous color change beside. (C) Another 2 months later, the mass enlarged with ulcerative surface after triamcinolone acetonide injections from the local clinic. 
|
Ultrasonography showed about 3-cm sized mass located in subcutaneous and a dermal layer with increased vascularity and inflammatory change, indicating a ruptured epidermal inclusion cyst (
Fig. 2). The patient was referred to us for mass excision to obtain pathologic diagnosis. On her second physical examination, the mass was more protruding than before and there was erythematous color change. We prescribed oral antibiotics and asked the patient to visit back 1 week later without surgical intervention; however, she didn’t show up. After 2 months, she came back with more protruding mass with ulcerative surface after other triamcinolone acetonide shot from the local clinic (
Fig. 1B,
1C).
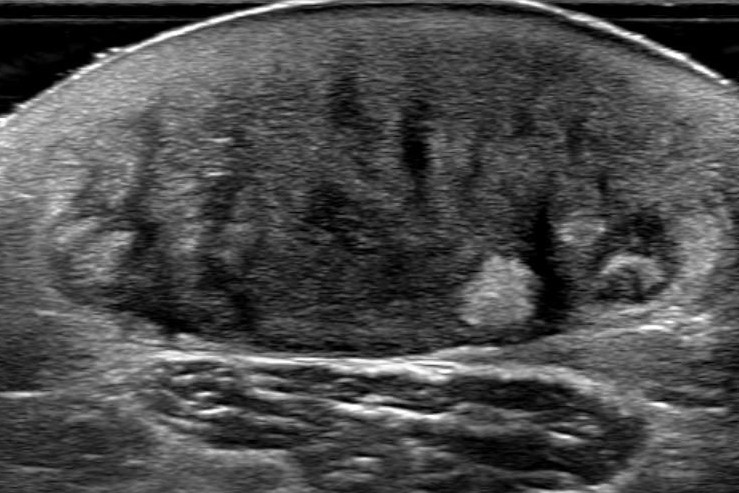 | Fig. 2.Ruptured epidermal inclusion body cyst was considered by ultrasonography. The mass was shown in ultrasonography at the lateral aspect of the right arm. The mass is heterogeneously intense with associated vascularity at a measured size of 3.0×1.5×3.0 cm. The image provided the possibility of ruptured epidermal inclusion body cyst due to its feature. 
|
For further evaluation of the mass, magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) was performed. MRI showed tissue mass in the dermal and subcutaneous layer with deltoid fascia involvement, highly suggestive dermatofibrosarcoma protuberans (
Fig. 3). PET-CT showed fluorine-18-fluorodeoxyglucose-avid malignancy mass in the right upper arm with suspected metastatic lymph in the right axilla. An incisional biopsy was performed, and histologic findings suggested a malignant tumor, likely squamous cell carcinoma with sarcomatoid differentiation. Before the surgery, the chest CT and bone scan were done. On chest CT, multiple prominent lymph node invasions were noticed in the right axilla. The bone scan showed no bone metastasis.
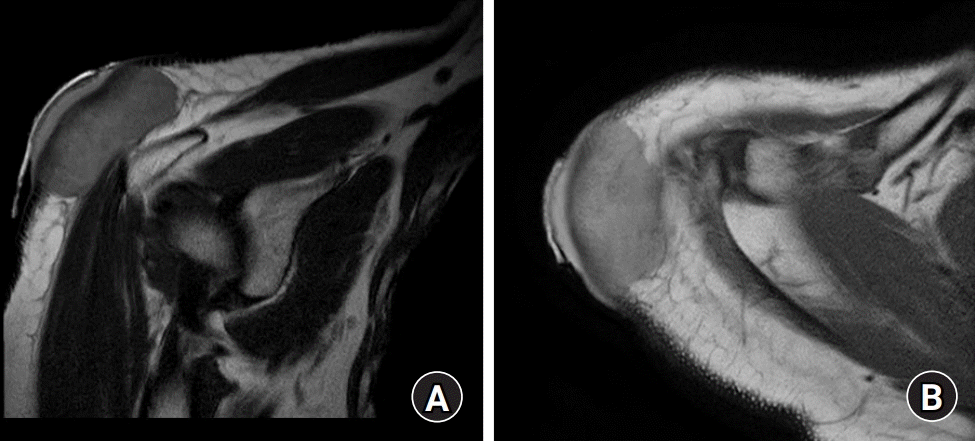 | Fig. 3.The mass sited in dermal and subcutaneous layer suggests deltoid fascia involvement. (A) The mass showed low intensity compared to the adjacent subcutaneous fat layer in a T1 image of magnetic resonance imaging (MRI). (B) Whereas the mass showed higher intensity than the surrounding dermal layer providing high suspicion of dermatofibrosarcoma protuberans in a T2 image of MRI. 
|
Right before operation,
99mTc-phytate lymphoscintigraphy was performed to detect the sentinel node. Tumor resection was performed by a marginal excision, including some deltoid muscle and soft tissue. The size of the excised mass was 15.7×13.5×1.6 cm. After mass excision, sentinel lymph node biopsy was performed using a gamma counter on suspected metastatic lymph nodes on the axilla. For the reconstruction of skin and soft tissue defect on the right upper arm, pedicled latissimus dorsi flap was performed on the lateral side of the defect (
Fig. 4).
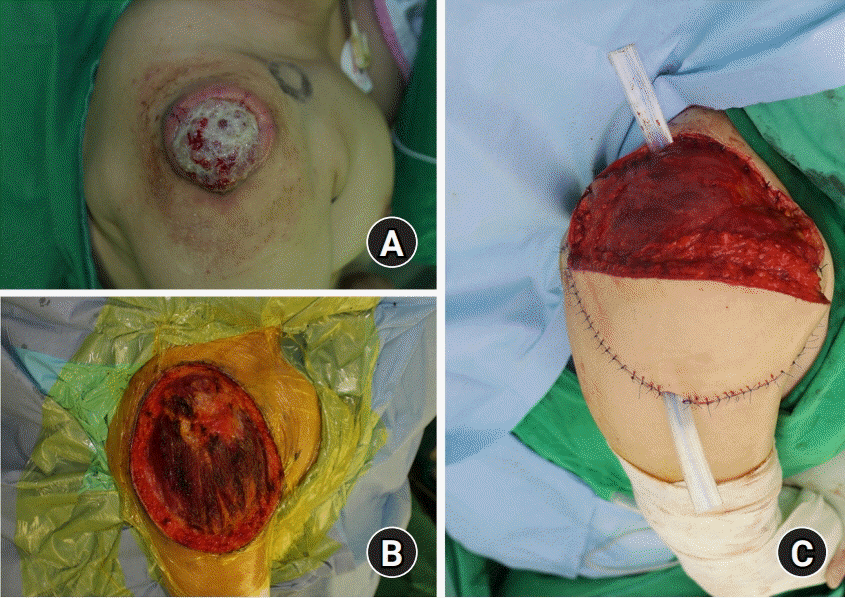 | Fig. 4.Intraoperative photographs. (A) Preoperative photo of mass. (B) About 17.5×14.0-cm sized defect on the right shoulder and upper arm. (C) Reconstruction using latissimus dorsi myocutaneous island flap on the lateral side of the defect. 
|
The patient had histological figures of IDCS containing spindle-like, oval shapes of cell and spherical or oval nucleoli (
Fig. 5). And immunohistochemical studies suggested IDCS since there was expression of S-100, vimentin, CD45, and the absence of melan A, smooth muscle actin (SMA), epithelial membrane antigen (EMA), CD34, CD1α, and CD21. Based on this, the patient was diagnosed with IDCS [
1,
6].
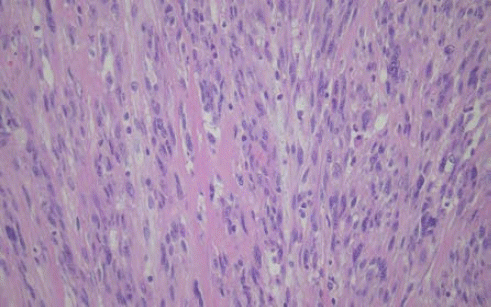 | Fig. 5.Microscopic examinations of interdigitating dendritic cell sarcoma (H&E staining, ×100). It was composed of spindle-like, oval-shaped cell with spherical or oval nucleoli. 
|
The skin graft was down 3 weeks later to cover the remaining defect (
Fig. 6). Since complete resection of mass and dissection of axillary lymph node was performed, additional adjuvant therapy was not administered.
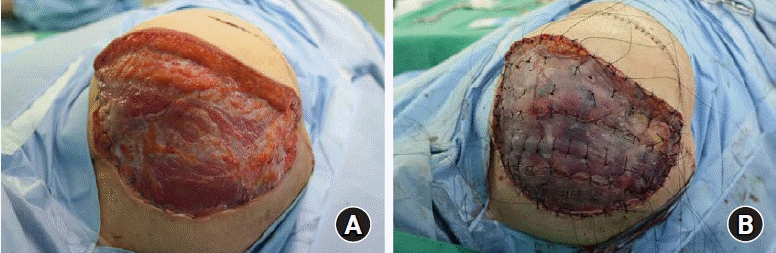 | Fig. 6.Intraoperative photographs. (A) The remaining skin and soft tissue defect on the right shoulder. (B) Reconstruction of the defect using split thickness skin graft. 
|
After surgery, the patient was under rehabilitation therapy for pain and decreased range of motion of the shoulder. And also, the patient underwent regular follow-up for recurrence. Nine months after surgery, the pain was almost gone and the range of motion was improved. Follow-up MRI and PET-CT revealed no tumor recurrence or metastasis (
Fig. 7). The requirements for approval of the Institutional Review Board were waived. Written informed consents was received from the patient for this report.
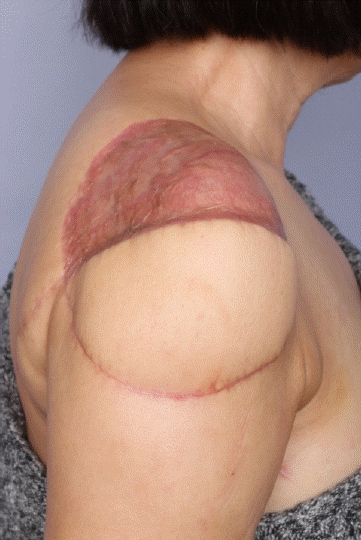 | Fig. 7.Nine months after the operation, the reconstruction site was stable and the patient was under rehabilitation therapy. 
|
Go to :

DISCUSSION
Dendritic cells are bone marrow-derived leukocytes; the most potent type of antigen-presenting cells. There are four types of dendritic cells in lymph nodes; follicular, interdigitating, Langerhans, and histiocytic/fibroblastic cells.
Dendritic cell neoplasm is rare and the tumor arising from interdigitating dendritic cells is extremely rare. Fewer than 200 cases have been reported. Furthermore, about one-third of IDCS occurs at extranodal such as liver, gastrointestinal tract, lung, spleen, skin, nasopharynx, and kidney. Its risk factors have not been identified yet [
1,
7,
8].
IDCS can be diagnosed based on histologic and immunohistochemical analysis. Histologically, large fusiform spindle cells with indistinct cell borders, oval central nuclei, finely dispersed chromatin, and small but prominent nucleoli, and often a storiform or whorled, fascicular growth pattern are usually found in IDCS. Immunohistochemically, IDCS is characterized by positive staining for S-100, vimentin, HLA-DR, or CD68, and negative staining for CD1α, CD21, CD35, CD3, or CD20.
In our case, the patient had histological figures of IDCS containing spindle-like, oval shapes of cell and spherical or oval nucleoli. Moreover, immunohistochemical studies also suggested IDCS since there was expression of S-100, vimentin, CD45, and the absence of melan A, SMA, EMA, CD34, CD1α, and CD21.
IDCS should be distinguished from follicular dendritic cell sarcoma, indeterminate dendritic cell tumor, Langerhans cell histiocytosis, and Langerhans cell sarcoma based on morphological and immunohistochemical properties [
9].
Generally, IDCS is known to have poor prognosis; however, due to the limited cases reported, there are no specific guidelines for the treatment of IDCS. Surgical excision is recommended for patients with localized disease, while postoperative adjuvant chemotherapy and radiotherapy still remain controversial. For patients with metastatic disease, chemotherapy followed by surgical excision is recommended. In some cases, combination chemotherapy was used for the patients such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), ICE (ifosfamide, carboplatin, etoposide), EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), and DHAP (dexamethasone, cisplatin, high-dose cytarabine), and had shown promising achievements though still lacking evidence-based significance. Radiation therapy, alone or with chemotherapy, has been used in some cases, but the role of radiotherapy has not been clearly identified [
6,
10].
This study reports a case study of a 66-year old female patient with IDCS on the skin with lymph node metastasis. At first, she was misdiagnosed with inflammation or ruptured epidermal inclusion cyst, which saw her being prescribed for local triamcinolone acetonide injection and antibiotic therapy. However, with no response from the prescriptions, further evaluation including biopsy was performed and finally the patient was diagnosed with malignancy. We found that the patient had lymph node metastasis thanks to excisional biopsy. However, it is uncertain that the metastasis of lymph nodes was undergone before her first visit or during follow-up period. After the complete resection of IDCS including axillary lymph nodes, there was no evidence of recurred malignancy with regards to the postoperative PET-CT, and the patient was not considered for additional adjuvant chemotherapy or radiotherapy. Although the patient appeared disease-free since surgery (5 months follow-up), careful monitoring for recurrence is necessary.
IDCS is an extremely rare neoplasm with limited cases reported. It usually arises in lymph nodes, but can also be found in extranodal sites. We report the case of IDCS found in the skin with axillary lymph node metastasis. Although its incidence is extremely rare, this case suggests that extranodal IDCS should be considered in differential diagnosis of untreated atypical skin mass and early biopsy should be performed.
Go to :








 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download