INTRODUCTION
VACCINATION STRATEGY OF COVID-19 IN KOREA
Table 1
Summary of the coronavirus disease 2019 vaccines introduced in the Republic of Korea
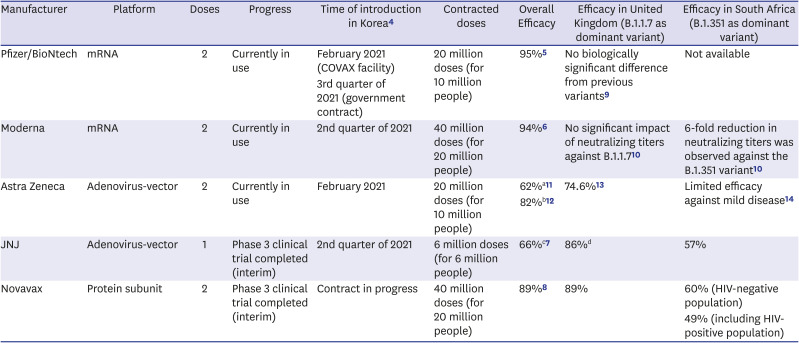
| Manufacturer | Platform | Doses | Progress | Time of introduction in Korea4 | Contracted doses | Overall Efficacy | Efficacy in United Kingdom (B.1.1.7 as dominant variant) | Efficacy in South Africa (B.1.351 as dominant variant) |
|---|---|---|---|---|---|---|---|---|
| Pfizer/BioNtech | mRNA | 2 | Currently in use | February 2021 (COVAX facility) | 20 million doses (for 10 million people) | 95%5 | No biologically significant difference from previous variants9 | Not available |
| 3rd quarter of 2021 (government contract) | ||||||||
| Moderna | mRNA | 2 | Currently in use | 2nd quarter of 2021 | 40 million doses (for 20 million people) | 94%6 | No significant impact of neutralizing titers against B.1.1.710 | 6-fold reduction in neutralizing titers was observed against the B.1.351 variant10 |
| Astra Zeneca | Adenovirus-vector | 2 | Currently in use | February 2021 | 20 million doses (for 10 million people) | 62%a11 | 74.6%13 | Limited efficacy against mild disease14 |
| 82%b12 | ||||||||
| JNJ | Adenovirus-vector | 1 | Phase 3 clinical trial completed (interim) | 2nd quarter of 2021 | 6 million doses (for 6 million people) | 66%c7 | 86%d | 57% |
| Novavax | Protein subunit | 2 | Phase 3 clinical trial completed (interim) | Contract in progress | 40 million doses (for 20 million people) | 89%8 | 89% | 60% (HIV-negative population) |
| 49% (including HIV-positive population) |
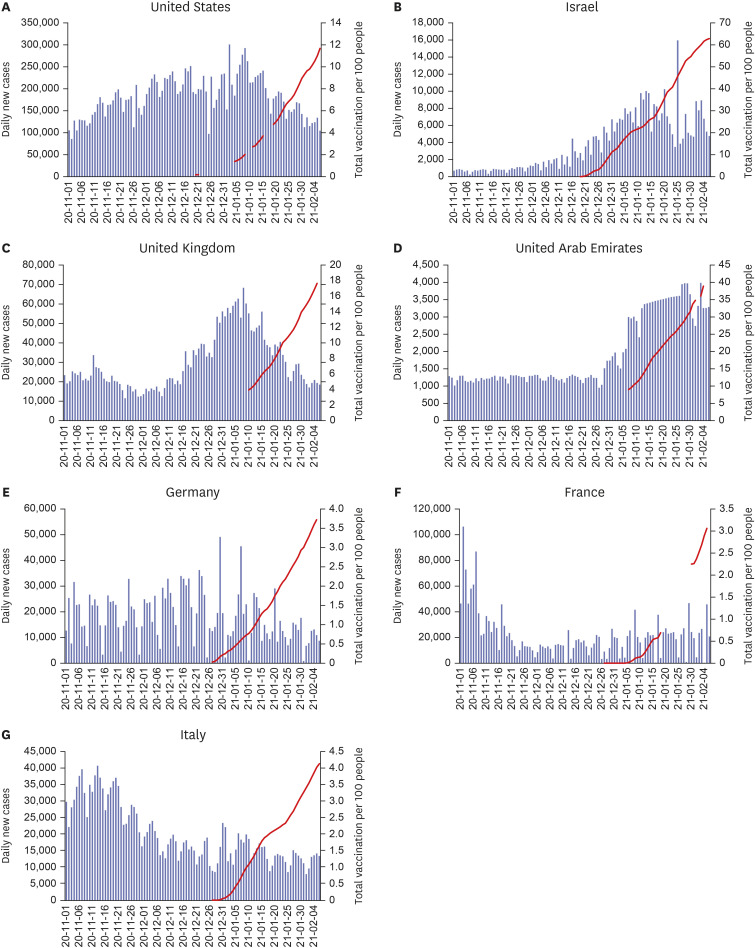
VACCINATION RATE AND EPIDEMIC TRENDS IN MAJOR COUNTRIES
PRIORITY SETTING OF VACCINATION
Fig. 3
Proportion of confirmed cases of coronavirus disease 2019 and deaths by age in the Republic of Korea (until January 31, 2021).
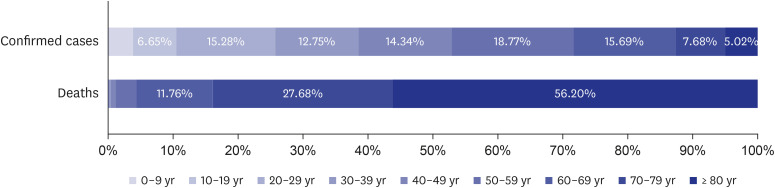
Table 2
Priorities for COVID-19 vaccination in major countries
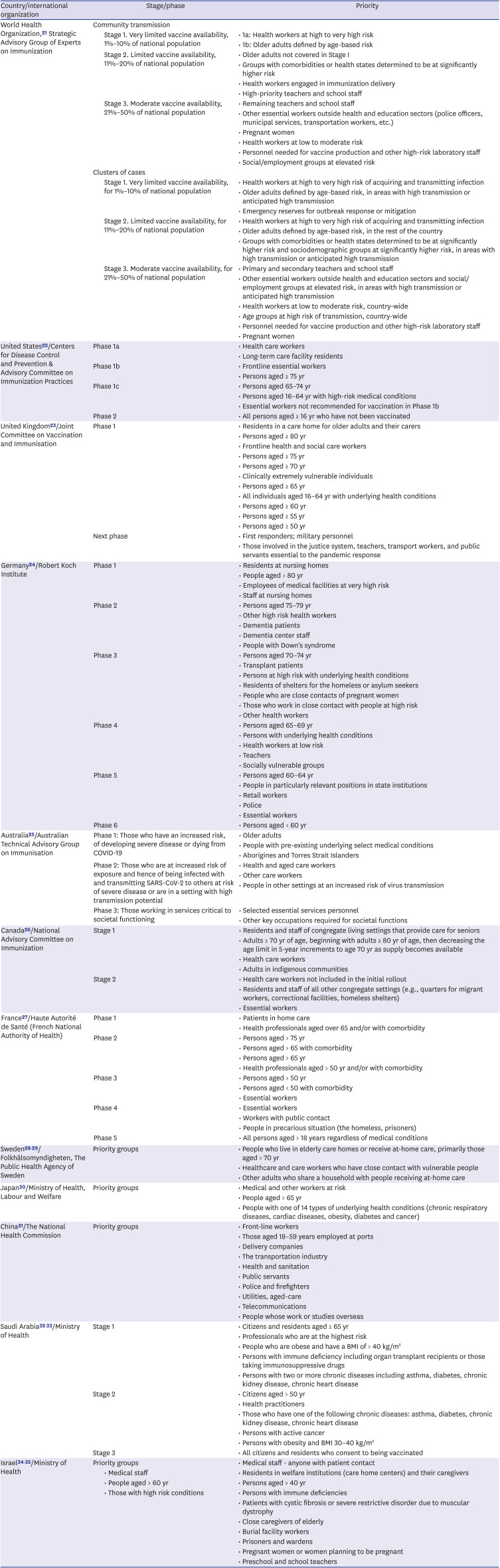
| Country/international organization | Stage/phase | Priority | |
|---|---|---|---|
| World Health Organization,21 Strategic Advisory Group of Experts on Immunization | Community transmission | ||
| Stage 1. Very limited vaccine availability, 1%–10% of national population | • 1a: Health workers at high to very high risk | ||
| • 1b: Older adults defined by age-based risk | |||
| Stage 2. Limited vaccine availability, 11%–20% of national population | • Older adults not covered in Stage I | ||
| • Groups with comorbidities or health states determined to be at significantly higher risk | |||
| • Health workers engaged in immunization delivery | |||
| • High-priority teachers and school staff | |||
| Stage 3. Moderate vaccine availability, 21%–50% of national population | • Remaining teachers and school staff | ||
| • Other essential workers outside health and education sectors (police officers, municipal services, transportation workers, etc.) | |||
| • Pregnant women | |||
| • Health workers at low to moderate risk | |||
| • Personnel needed for vaccine production and other high-risk laboratory staff | |||
| • Social/employment groups at elevated risk | |||
| Clusters of cases | |||
| Stage 1. Very limited vaccine availability, for 1%–10% of national population | • Health workers at high to very high risk of acquiring and transmitting infection | ||
| • Older adults defined by age-based risk, in areas with high transmission or anticipated high transmission | |||
| • Emergency reserves for outbreak response or mitigation | |||
| Stage 2. Limited vaccine availability, for 11%–20% of national population | • Health workers at high to very high risk of acquiring and transmitting infection | ||
| • Older adults defined by age-based risk, in the rest of the country | |||
| • Groups with comorbidities or health states determined to be at significantly higher risk and sociodemographic groups at significantly higher risk, in areas with high transmission or anticipated high transmission | |||
| Stage 3. Moderate vaccine availability, for 21%–50% of national population | • Primary and secondary teachers and school staff | ||
| • Other essential workers outside health and education sectors and social/employment groups at elevated risk, in areas with high transmission or anticipated high transmission | |||
| • Health workers at low to moderate risk, country-wide | |||
| • Age groups at high risk of transmission, country-wide | |||
| • Personnel needed for vaccine production and other high-risk laboratory staff | |||
| • Pregnant women | |||
| United States22/Centers for Disease Control and Prevention & Advisory Committee on Immunization Practices | Phase 1a | • Health care workers | |
| • Long-term care facility residents | |||
| Phase 1b | • Frontline essential workers | ||
| • Persons aged ≥ 75 yr | |||
| Phase 1c | • Persons aged 65–74 yr | ||
| • Persons aged 16–64 yr with high-risk medical conditions | |||
| • Essential workers not recommended for vaccination in Phase 1b | |||
| Phase 2 | • All persons aged ≥ 16 yr who have not been vaccinated | ||
| United Kingdom23/Joint Committee on Vaccination and Immunisation | Phase 1 | • Residents in a care home for older adults and their carers | |
| • Persons aged ≥ 80 yr | |||
| • Frontline health and social care workers | |||
| • Persons aged ≥ 75 yr | |||
| • Persons aged ≥ 70 yr | |||
| • Clinically extremely vulnerable individuals | |||
| • Persons aged ≥ 65 yr | |||
| • All individuals aged 16–64 yr with underlying health conditions | |||
| • Persons aged ≥ 60 yr | |||
| • Persons aged ≥ 55 yr | |||
| • Persons aged ≥ 50 yr | |||
| Next phase | • First responders; military personnel | ||
| • Those involved in the justice system, teachers, transport workers, and public servants essential to the pandemic response | |||
| Germany24/Robert Koch Institute | Phase 1 | • Residents at nursing homes | |
| • People aged > 80 yr | |||
| • Employees of medical facilities at very high risk | |||
| • Staff at nursing homes | |||
| Phase 2 | • Persons aged 75–79 yr | ||
| • Other high risk health workers | |||
| • Dementia patients | |||
| • Dementia center staff | |||
| • People with Down's syndrome | |||
| Phase 3 | • Persons aged 70–74 yr | ||
| • Transplant patients | |||
| • Persons at high risk with underlying health conditions | |||
| • Residents of shelters for the homeless or asylum seekers | |||
| • People who are close contacts of pregnant women | |||
| • Those who work in close contact with people at high risk | |||
| • Other health workers | |||
| Phase 4 | • Persons aged 65–69 yr | ||
| • Persons with underlying health conditions | |||
| • Health workers at low risk | |||
| • Teachers | |||
| • Socially vulnerable groups | |||
| Phase 5 | • Persons aged 60–64 yr | ||
| • People in particularly relevant positions in state institutions | |||
| • Retail workers | |||
| • Police | |||
| • Essential workers | |||
| Phase 6 | • Persons aged < 60 yr | ||
| Australia25/Australian Technical Advisory Group on Immunisation | Phase 1: Those who have an increased risk, of developing severe disease or dying from COVID-19 | • Older adults | |
| • People with pre-existing underlying select medical conditions | |||
| • Aborigines and Torres Strait Islanders | |||
| Phase 2: Those who are at increased risk of exposure and hence of being infected with and transmitting SARS-CoV-2 to others at risk of severe disease or are in a setting with high transmission potential | • Health and aged care workers | ||
| • Other care workers | |||
| • People in other settings at an increased risk of virus transmission | |||
| Phase 3: Those working in services critical to societal functioning | • Selected essential services personnel | ||
| • Other key occupations required for societal functions | |||
| Canada26/National Advisory Committee on Immunization | Stage 1 | • Residents and staff of congregate living settings that provide care for seniors | |
| • Adults ≥ 70 yr of age, beginning with adults ≥ 80 yr of age, then decreasing the age limit in 5-year increments to age 70 yr as supply becomes available | |||
| • Health care workers | |||
| • Adults in indigenous communities | |||
| Stage 2 | • Health care workers not included in the initial rollout | ||
| • Residents and staff of all other congregate settings (e.g., quarters for migrant workers, correctional facilities, homeless shelters) | |||
| • Essential workers | |||
| France27/Haute Autorité de Santé (French National Authority of Health) | Phase 1 | • Patients in home care | |
| • Health professionals aged over 65 and/or with comorbidity | |||
| Phase 2 | • Persons aged > 75 yr | ||
| • Persons aged > 65 with comorbidity | |||
| • Persons aged > 65 yr | |||
| • Health professionals aged > 50 yr and/or with comorbidity | |||
| Phase 3 | • Persons aged > 50 yr | ||
| • Persons aged < 50 with comorbidity | |||
| • Essential workers | |||
| Phase 4 | • Essential workers | ||
| • Workers with public contact | |||
| • People in precarious situation (the homeless, prisoners) | |||
| Phase 5 | • All persons aged > 18 years regardless of medical conditions | ||
| Sweden2829/Folkhälsomyndigheten, The Public Health Agency of Sweden | Priority groups | • People who live in elderly care homes or receive at-home care, primarily those aged > 70 yr | |
| • Healthcare and care workers who have close contact with vulnerable people | |||
| • Other adults who share a household with people receiving at-home care | |||
| Japan30/Ministry of Health, Labour and Welfare | Priority groups | • Medical and other workers at risk | |
| • People aged > 65 yr | |||
| • People with one of 14 types of underlying health conditions (chronic respiratory diseases, cardiac diseases, obesity, diabetes and cancer) | |||
| China31/The National Health Commission | Priority groups | • Front-line workers | |
| • Those aged 18–59 years employed at ports | |||
| • Delivery companies | |||
| • The transportation industry | |||
| • Health and sanitation | |||
| • Public servants | |||
| • Police and firefighters | |||
| • Utilities, aged-care | |||
| • Telecommunications | |||
| • People whose work or studies overseas | |||
| Saudi Arabia3233/Ministry of Health | Stage 1 | • Citizens and residents aged ≥ 65 yr | |
| • Professionals who are at the highest risk | |||
| • People who are obese and have a BMI of > 40 kg/m2 | |||
| • Persons with immune deficiency including organ transplant recipients or those taking immunosuppressive drugs | |||
| • Persons with two or more chronic diseases including asthma, diabetes, chronic kidney disease, chronic heart disease | |||
| Stage 2 | • Citizens aged > 50 yr | ||
| • Health practitioners | |||
| • Those who have one of the following chronic diseases: asthma, diabetes, chronic kidney disease, chronic heart disease | |||
| • Persons with active cancer | |||
| • Persons with obesity and BMI 30–40 kg/m2 | |||
| Stage 3 | • All citizens and residents who consent to being vaccinated | ||
| Israel3435/Ministry of Health | Priority groups | • Medical staff - anyone with patient contact | |
| • Medical staff | • Residents in welfare institutions (care home centers) and their caregivers | ||
| • People aged > 60 yr | • Persons aged > 40 yr | ||
| • Those with high risk conditions | • Persons with immune deficiencies | ||
| • Patients with cystic fibrosis or severe restrictive disorder due to muscular dystrophy | |||
| • Close caregivers of elderly | |||
| • Burial facility workers | |||
| • Prisoners and wardens | |||
| • Pregnant women or women planning to be pregnant | |||
| • Preschool and school teachers | |||
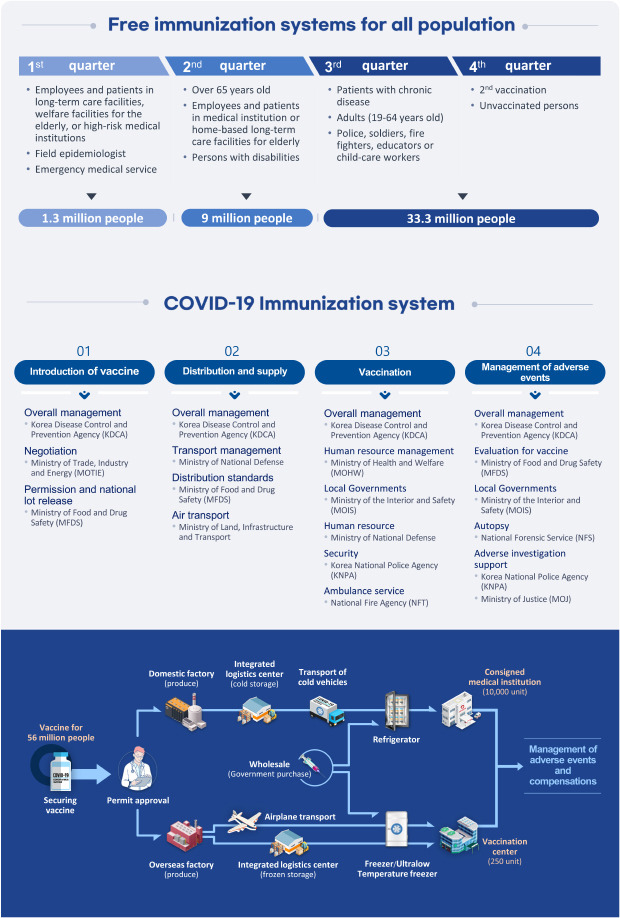
DELIVERY AND LOGISTICS OF VACCINATION
Table 3
COVID-19 vaccination facilities in major countries
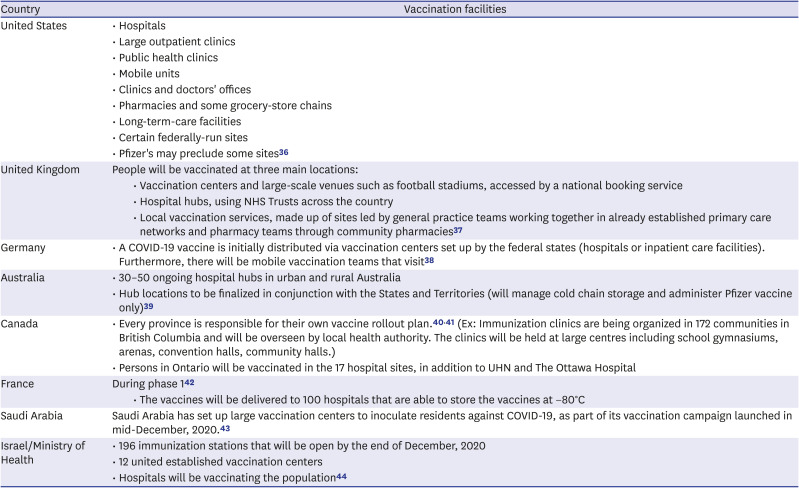
| Country | Vaccination facilities | |
|---|---|---|
| United States | • Hospitals | |
| • Large outpatient clinics | ||
| • Public health clinics | ||
| • Mobile units | ||
| • Clinics and doctors' offices | ||
| • Pharmacies and some grocery-store chains | ||
| • Long-term-care facilities | ||
| • Certain federally-run sites | ||
| • Pfizer's may preclude some sites36 | ||
| United Kingdom | People will be vaccinated at three main locations: | |
| • Vaccination centers and large-scale venues such as football stadiums, accessed by a national booking service | ||
| • Hospital hubs, using NHS Trusts across the country | ||
| • Local vaccination services, made up of sites led by general practice teams working together in already established primary care networks and pharmacy teams through community pharmacies37 | ||
| Germany | • A COVID-19 vaccine is initially distributed via vaccination centers set up by the federal states (hospitals or inpatient care facilities). Furthermore, there will be mobile vaccination teams that visit38 | |
| Australia | • 30–50 ongoing hospital hubs in urban and rural Australia | |
| • Hub locations to be finalized in conjunction with the States and Territories (will manage cold chain storage and administer Pfizer vaccine only)39 | ||
| Canada | • Every province is responsible for their own vaccine rollout plan.4041 (Ex: Immunization clinics are being organized in 172 communities in British Columbia and will be overseen by local health authority. The clinics will be held at large centres including school gymnasiums, arenas, convention halls, community halls.) | |
| • Persons in Ontario will be vaccinated in the 17 hospital sites, in addition to UHN and The Ottawa Hospital | ||
| France | During phase 142 | |
| • The vaccines will be delivered to 100 hospitals that are able to store the vaccines at −80°C | ||
| Saudi Arabia | Saudi Arabia has set up large vaccination centers to inoculate residents against COVID-19, as part of its vaccination campaign launched in mid-December, 2020.43 | |
| Israel/Ministry of Health | • 196 immunization stations that will be open by the end of December, 2020 | |
| • 12 united established vaccination centers | ||
| • Hospitals will be vaccinating the population44 | ||
Table 4
Vaccine distribution in major countries

| Countries | Vaccine | Storage, Transport, Logistics | |
|---|---|---|---|
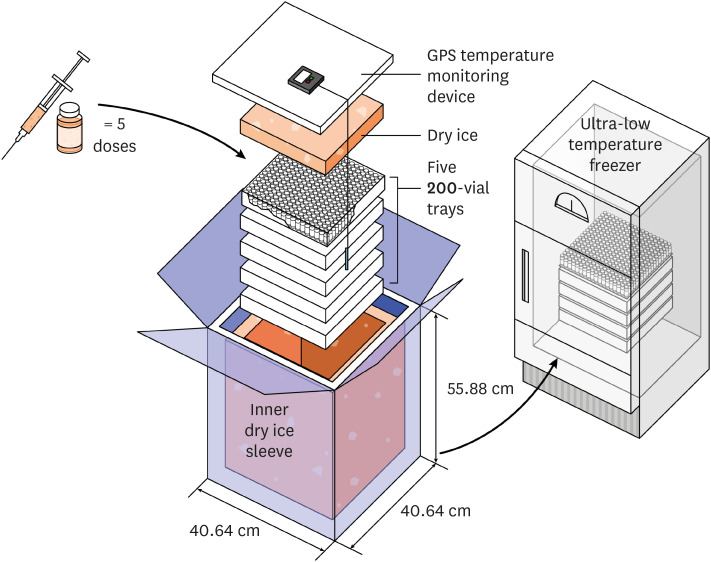
| Overall | Pfizer/BioNTech | Thermal shipper (−80°C to −70°C) for up to 6 mon, 5 days after transfer to refrigerator | |
| Moderna | Freezer (−20°C) for up to 6 mon, up to 30 days | ||
| AstraZeneca | Refrigerator (2°C–8°C) for up to 6 mon | ||
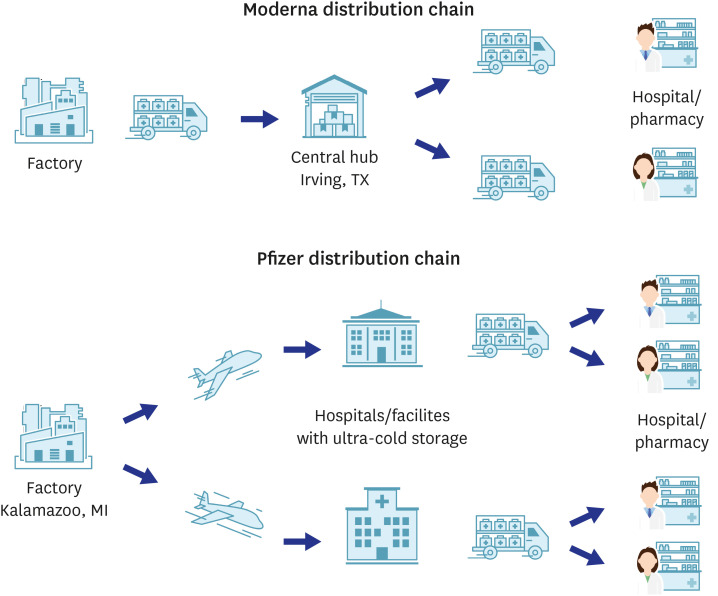
| United States4546 | Manufacturing factory: Kalamazoo, MI (Pfizer), Central Hub: Irving, Tx (Moderna) | ||
| Logistic partners: FedEx, UPS | |||
| • FedEx and UPS are each responsible for one half of the country | |||
| • Both UPS and FedEx will use high-tech tracking devices to monitor packages carrying the vaccines | |||
| • Pfizer has also installed its own tracking system on these boxes | |||
| • Vaccine shipments will be getting special treatment, including priority access at the airport | |||
| Australia47 | Logistic partners: DHL and Linfox | ||
| • Support vaccination for all, including people in rural and remote areas | |||
| • Required to track and report the temperature of the vaccines at all times | |||
| • Logistics partners will be responsible for transport and management of vaccination supplies such as needles, syringes, and personal protective equipment | |||
| Canada48 | An immunization NOC has been established as part of federal logistical coordination | ||
| • Supported by a multidisciplinary team of experts, including the Canadian Armed Forces | |||
| • FedEx Express Canada and Innomar Strategies will support the NOC with logistics and vaccines | |||
CONCERNS RELATED TO VACCINATION
Anaphylaxis after vaccination
Missed second dose
Flu-like symptom after vaccination
Taking antipyretic analgesics before vaccination
Vaccinations for recovery after COVID-19 infection
Need for SARS-CoV-2 diagnostic test before vaccination
Simultaneous vaccination with non-COVID-19 vaccines
Vaccine safety monitoring
Table 5
Vaccine safety monitoring and management in major countries
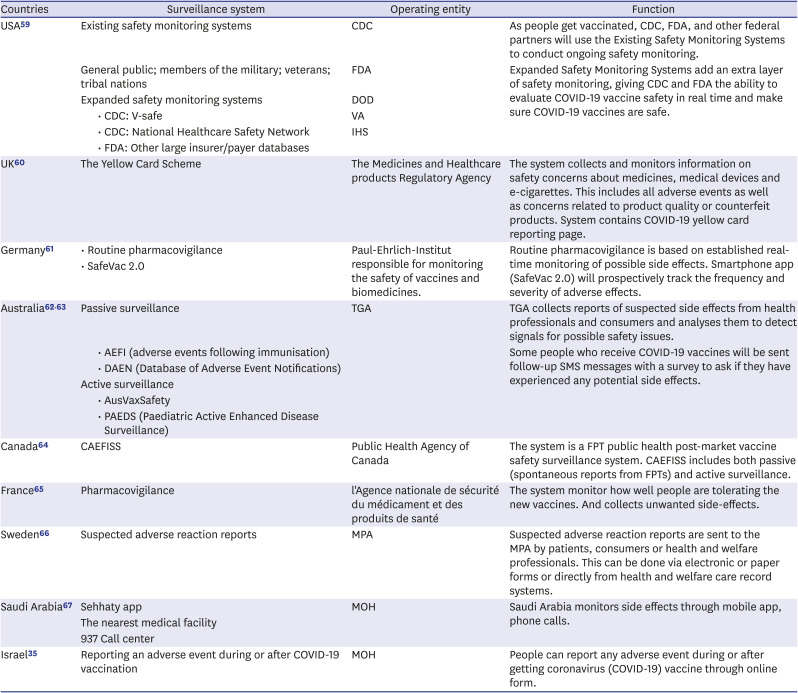
| Countries | Surveillance system | Operating entity | Function | |
|---|---|---|---|---|
| USA59 | Existing safety monitoring systems | CDC | As people get vaccinated, CDC, FDA, and other federal partners will use the Existing Safety Monitoring Systems to conduct ongoing safety monitoring. | |
| General public; members of the military; veterans; tribal nations | FDA | Expanded Safety Monitoring Systems add an extra layer of safety monitoring, giving CDC and FDA the ability to evaluate COVID-19 vaccine safety in real time and make sure COVID-19 vaccines are safe. | ||
| Expanded safety monitoring systems | DOD | |||
| • CDC: V-safe | VA | |||
| • CDC: National Healthcare Safety Network | IHS | |||
| • FDA: Other large insurer/payer databases | ||||
| UK60 | The Yellow Card Scheme | The Medicines and Healthcare products Regulatory Agency | The system collects and monitors information on safety concerns about medicines, medical devices and e-cigarettes. This includes all adverse events as well as concerns related to product quality or counterfeit products. System contains COVID-19 yellow card reporting page. | |
| Germany61 | • Routine pharmacovigilance | Paul-Ehrlich-Institut responsible for monitoring the safety of vaccines and biomedicines. | Routine pharmacovigilance is based on established real-time monitoring of possible side effects. Smartphone app (SafeVac 2.0) will prospectively track the frequency and severity of adverse effects. | |
| • SafeVac 2.0 | ||||
| Australia6263 | Passive surveillance | TGA | TGA collects reports of suspected side effects from health professionals and consumers and analyses them to detect signals for possible safety issues. | |
| • AEFI (adverse events following immunisation) | Some people who receive COVID-19 vaccines will be sent follow-up SMS messages with a survey to ask if they have experienced any potential side effects. | |||
| • DAEN (Database of Adverse Event Notifications) | ||||
| Active surveillance | ||||
| • AusVaxSafety | ||||
| • PAEDS (Paediatric Active Enhanced Disease Surveillance) | ||||
| Canada64 | CAEFISS | Public Health Agency of Canada | The system is a FPT public health post-market vaccine safety surveillance system. CAEFISS includes both passive (spontaneous reports from FPTs) and active surveillance. | |
| France65 | Pharmacovigilance | l'Agence nationale de sécurité du médicament et des produits de santé | The system monitor how well people are tolerating the new vaccines. And collects unwanted side-effects. | |
| Sweden66 | Suspected adverse reaction reports | MPA | Suspected adverse reaction reports are sent to the MPA by patients, consumers or health and welfare professionals. This can be done via electronic or paper forms or directly from health and welfare care record systems. | |
| Saudi Arabia67 | Sehhaty app | MOH | Saudi Arabia monitors side effects through mobile app, phone calls. | |
| The nearest medical facility | ||||
| 937 Call center | ||||
| Israel35 | Reporting an adverse event during or after COVID-19 vaccination | MOH | People can report any adverse event during or after getting coronavirus (COVID-19) vaccine through online form. | |
CHANGES AFTER STARTING VACCINATION
Virus variants
Non-pharmaceutical interventions
Table 6
Financial support for affected sectors in different countries
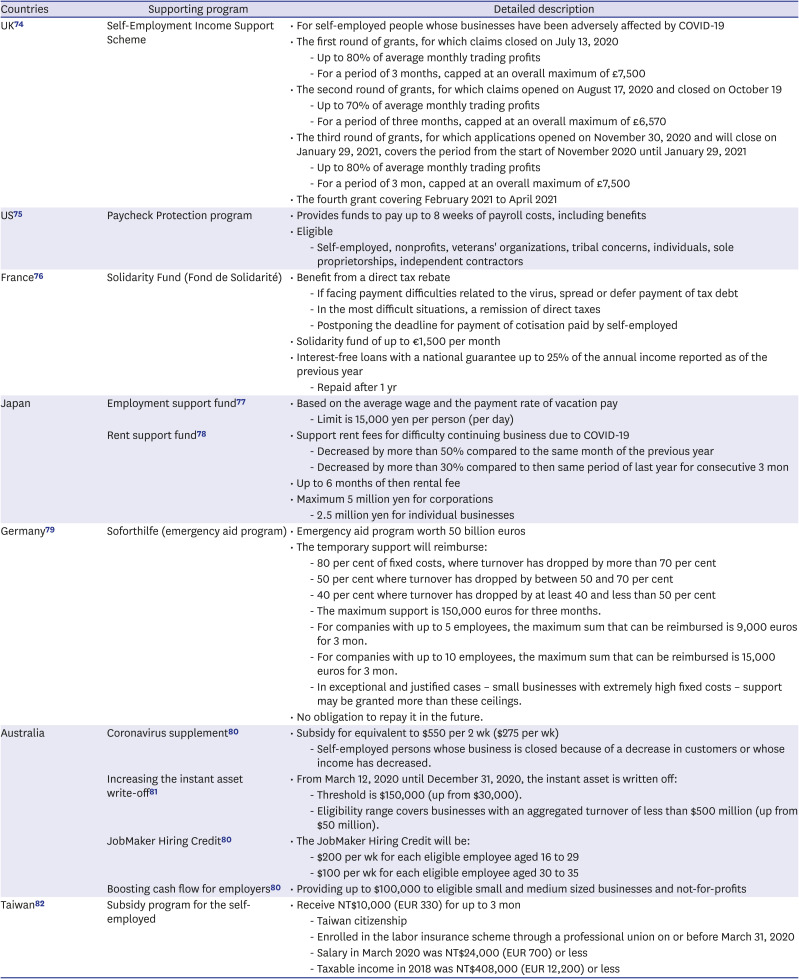
| Countries | Supporting program | Detailed description | |
|---|---|---|---|
| UK74 | Self-Employment Income Support Scheme | • For self-employed people whose businesses have been adversely affected by COVID-19 | |
| • The first round of grants, for which claims closed on July 13, 2020 | |||
| - Up to 80% of average monthly trading profits | |||
| - For a period of 3 months, capped at an overall maximum of £7,500 | |||
| • The second round of grants, for which claims opened on August 17, 2020 and closed on October 19 | |||
| - Up to 70% of average monthly trading profits | |||
| - For a period of three months, capped at an overall maximum of £6,570 | |||
| • The third round of grants, for which applications opened on November 30, 2020 and will close on January 29, 2021, covers the period from the start of November 2020 until January 29, 2021 | |||
| - Up to 80% of average monthly trading profits | |||
| - For a period of 3 mon, capped at an overall maximum of £7,500 | |||
| • The fourth grant covering February 2021 to April 2021 | |||
| US75 | Paycheck Protection program | • Provides funds to pay up to 8 weeks of payroll costs, including benefits | |
| • Eligible | |||
| - Self-employed, nonprofits, veterans' organizations, tribal concerns, individuals, sole proprietorships, independent contractors | |||
| France76 | Solidarity Fund (Fond de Solidarité) | • Benefit from a direct tax rebate | |
| - If facing payment difficulties related to the virus, spread or defer payment of tax debt | |||
| - In the most difficult situations, a remission of direct taxes | |||
| - Postponing the deadline for payment of cotisation paid by self-employed | |||
| • Solidarity fund of up to €1,500 per month | |||
| • Interest-free loans with a national guarantee up to 25% of the annual income reported as of the previous year | |||
| - Repaid after 1 yr | |||
| Japan | Employment support fund77 | • Based on the average wage and the payment rate of vacation pay | |
| - Limit is 15,000 yen per person (per day) | |||
| Rent support fund78 | • Support rent fees for difficulty continuing business due to COVID-19 | ||
| - Decreased by more than 50% compared to the same month of the previous year | |||
| - Decreased by more than 30% compared to then same period of last year for consecutive 3 mon | |||
| • Up to 6 months of then rental fee | |||
| • Maximum 5 million yen for corporations | |||
| - 2.5 million yen for individual businesses | |||
| Germany79 | Soforthilfe (emergency aid program) | • Emergency aid program worth 50 billion euros | |
| • The temporary support will reimburse: | |||
| - 80 per cent of fixed costs, where turnover has dropped by more than 70 per cent | |||
| - 50 per cent where turnover has dropped by between 50 and 70 per cent | |||
| - 40 per cent where turnover has dropped by at least 40 and less than 50 per cent | |||
| - The maximum support is 150,000 euros for three months. | |||
| - For companies with up to 5 employees, the maximum sum that can be reimbursed is 9,000 euros for 3 mon. | |||
| - For companies with up to 10 employees, the maximum sum that can be reimbursed is 15,000 euros for 3 mon. | |||
| - In exceptional and justified cases – small businesses with extremely high fixed costs – support may be granted more than these ceilings. | |||
| • No obligation to repay it in the future. | |||
| Australia | Coronavirus supplement80 | • Subsidy for equivalent to $550 per 2 wk ($275 per wk) | |
| - Self-employed persons whose business is closed because of a decrease in customers or whose income has decreased. | |||
| Increasing the instant asset write-off81 | • From March 12, 2020 until December 31, 2020, the instant asset is written off: | ||
| - Threshold is $150,000 (up from $30,000). | |||
| - Eligibility range covers businesses with an aggregated turnover of less than $500 million (up from $50 million). | |||
| JobMaker Hiring Credit80 | • The JobMaker Hiring Credit will be: | ||
| - $200 per wk for each eligible employee aged 16 to 29 | |||
| - $100 per wk for each eligible employee aged 30 to 35 | |||
| Boosting cash flow for employers80 | • Providing up to $100,000 to eligible small and medium sized businesses and not-for-profits | ||
| Taiwan82 | Subsidy program for the self-employed | • Receive NT$10,000 (EUR 330) for up to 3 mon | |
| - Taiwan citizenship | |||
| - Enrolled in the labor insurance scheme through a professional union on or before March 31, 2020 | |||
| - Salary in March 2020 was NT$24,000 (EUR 700) or less | |||
| - Taxable income in 2018 was NT$408,000 (EUR 12,200) or less | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download