Abstract
Mesenchymal stem cells (MSCs) represent a population of adult stem cells residing in many tissues, mainly bone marrow, adipose tissue, and umbilical cord. Due to the safety and availability of standard procedures and protocols for isolation, culturing, and characterization of these cells, MSCs have emerged as one of the most promising sources for cell-based cardiac regenerative therapy. Once transplanted into a damaged heart, MSCs release paracrine factors that nurture the injured area, prevent further adverse cardiac remodeling, and mediate tissue repair along with vasculature. Numerous preclinical studies applying MSCs have provided significant benefits following myocardial infarction. Despite promising results from preclinical studies using animal models, MSCs are not up to the mark for human clinical trials. As a result, various approaches have been considered to promote the therapeutic potency of MSCs, such as genetic engineering, physical treatments, growth factor, and pharmacological agents. Each strategy has targeted one or multi-potentials of MSCs. In this review, we will describe diverse approaches that have been developed to promote the therapeutic potential of MSCs for cardiac regenerative therapy. Particularly, we will discuss major characteristics of individual strategy to enhance therapeutic efficacy of MSCs including scientific principles, advantages, limitations, and improving factors. This article also will briefly introduce recent novel approaches that MSCs enhanced therapeutic potentials of other cells for cardiac repair.
Heart failure is one of major causes of hospitalization worldwide. It has been estimated that 1–2% of adult population are currently suffering from heart diseases.1)2) Heart failure is the condition in which the heart fails to pump sufficient volume of blood to meet the body's demand due to substantial reduction in the contractile function of the myocardium.1)2) Despite its clinical importance, current regimens including surgical and pharmacological interventions can only delay the progression of this detrimental disease.
Due to extremely low regeneration potential, heart transplantation is one of a few currently available treatment options for improving the prognosis of this disease. However, it is hampered by the limited supply of donor hearts and transplant-related immunosuppressant therapy. An artificial heart device can be used as a bridge to transplantation for patients waiting for heart donors. However, artificial heart devices have certain limitations, including unknown durability, the use of anticoagulants, and post-surgical complications.3) Due to restricted therapeutic options and the least regenerative nature of heart tissue, researchers have long been looking for alternative options to compensate damaged heart tissues.4)5) As a result, cell-based cardiac regeneration therapy has emerged as one of the most promising alternatives for treating damaged hearts.6)
Several distinct cell types are sources for cardiac regeneration therapy, including human mesenchymal stem cells (hMSCs),7) adipose tissue-derived stem cells,8) umbilical cord-derived stem cells,9) skeletal muscle derived stem cells,10) and residential cardiac stem cells.11) All these cell-based therapeutic strategies aim to reduce myocardial damage by regenerating new functional cardiac tissues.12)13) Among these cells, hMSCs present a valuable option for cardiac regenerative therapy because of their ability to engraft and differentiate inside a heart with paracrine signaling, homing abilities. In addition, they can confer immunosuppression.14)15) hMSCs represent a population of multipotent stem cells present in adult tissues such as bone marrow, adipose tissue, umbilical cord, and peripheral blood. These cells can be easily isolated from their source tissues and suitably expanded in culture for several passages without any change in differentiating abilities. They are characterized by their adherence properties, expression of certain cell surface markers, and differentiation potential to a variety of cell lineages like osteoblasts, chondroblasts, and adipocytes. Additionally, several studies have suggested that hMSCs possess phenotypic characteristics of other cell types such as endothelial cells, smooth muscle, neural, skeletal, and cardiac myoblasts.14)16) Due to these advantages, hMSCs are promising candidates for allogeneic transplantation as they do not express MHC class II molecule or Fas ligand. Moreover, hMSCs have a unique capability of reaching the injured site without requiring any homing device.17)18) Upon introduction into an infarcted heart, mesenchymal stem cells (MSCs) have the potential to prevent further deleterious remodeling and improve cardiac function. Mechanisms of transplanted MSCs include differentiation of MSCs into cardiomyocyte-like cells19) as well as paracrine effects that include modulating antifibrotic environment,20) immunomodulating effect,21) improving resistance against apoptosis,22) increasing cytoprotective effect,23) and enhancing angiogenesis.24) Compared to direct differentiation of MSCs into cardiomyocytes, paracrine signaling is strongly focused and widely accepted.25) A plethora of data have suggested that MSCs can release cytokines, chemokines, growth factors, and microRNA to harmonize various cells and improve the microenvironment of injured hearts.26)27) In line with this, Korf-Klingebiel et al.28) have demonstrated that hMSCs can release trophic factors and pro-angiogenic factors such as FGF9, VEGF, BMP2, DKK1, CXCL12, JAG1, WISP, and INHBA. Those factors not only can increase cardiomyocyte survival after myocardial infarction, but also can enhance angiogenesis. Therefore, MSCs is a distinct source of paracrine signaling for cardiovascular repair.
Several preclinical studies using animal models have demonstrated that administration of hMSCs into the damaged area is safe. In addition, it can decrease the infarct size and improve contractile function with better survival post-engraftment.29)30) Following positive results in animal models, many groups have conducted clinical trials for MSCs.31) Some of these trials have demonstrated improvement of left ventricular ejection function (LVEF).32)33)34)35) For example, the BOOST trial has shown that LVEF in the hMSCs treatment group is enhanced 6.7% while LVEF in the control group is increased only 0.7% after 6 months.36) However, a meta-analysis study based on individual patient data of 1,252 patients has demonstrated no benefit from bone marrow-derived cells for myocardial infarction (MI) treatment in terms of LVEF or clinical events.37) Accordingly, a summary of 17 clinical trials applying bone marrow MSCs has revealed that only 10/17 studies show positive effects on LVEF change.38) Some other groups have also reported positive results. However, results of these studies were inconclusive due to the lack of control groups.39)40)41) In conclusion, most clinical trials did not report any significant improvement in left ventricle function after the administration of MSCs. Although outcomes from these efforts were minimal and most clinical trials failed to demonstrate an accurate and measurable improvement in cardiac function, they enhanced our knowledge regarding hMSC therapy and the underlying mechanism that paved a way for further improvements.13)
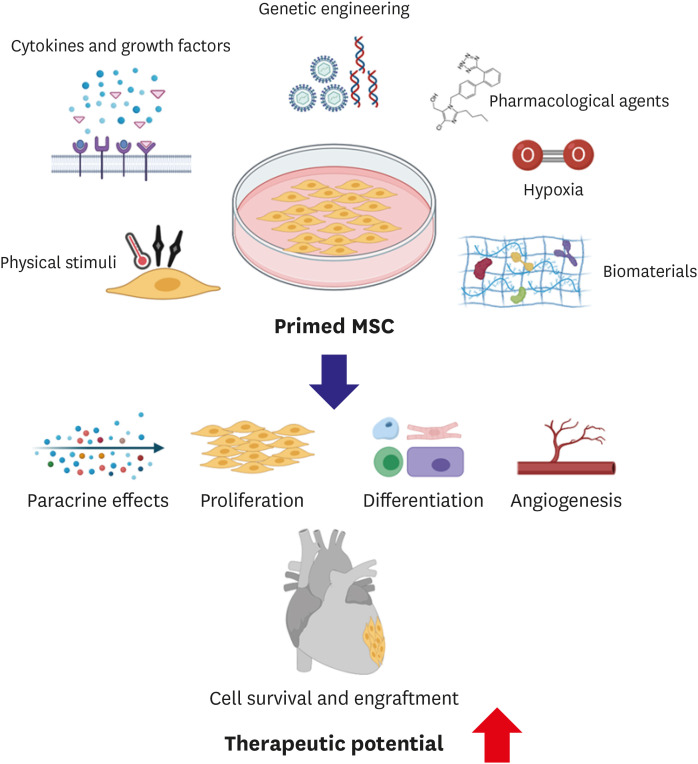
To improve the therapeutic potency of hMSCs, multi-faceted approaches have been developed during the last two decades (Figure 1).38)42) Notably, there is a growing evidence that priming of MSCs with diverse strategies under in vitro condition prior to transplantation can promote therapeutic potentials of MSCs substantially through enhanced survival, retention, and engraftment of MSCs post transplantation into the heart. Priming of MSCs can be achieved by a number of applications, including exposing MSCs to hypoxic or anoxic conditions, treating with numerous pharmacological/chemical agents,43) cytokines, or growth factors,44) and using physical stimuli including biomaterials.45)46)47)48) In addition to these mentioned benefits of MSCs, some studies have found that MSCs can enhance the therapeutic potential of other cell types, including cardiomyocytes.49) Thus, in this review article, we will discuss several strategies previously attempted to enhance the therapeutic potency of hMSCs. We will also discuss MSCs-based novel strategies that can significantly enhance cardiac function post MI.
One of the first strategies to enhance therapeutic potentials of hMSCs is genetic engineering to modulate the expression of genes of interest through non-viral or viral vectors. These genetic modifications enable hMSCs to express certain proteins, cytokines, enzymes, and cell surface molecules necessary for their survival, migration, adhesion, and angiogenesis.17)38) Overexpression of a pro-survival serine-threonine kinase gene Akt confers cytoprotective effects of hMSCs. Transplantation of these genetically modified MSCs into ischemic hearts can restore myocardium, inhibit adverse cardiac remodeling, significantly improve LVEF, and reduce MI site.50)51) Transduced MSCs also show higher resistance to apoptosis along with enhanced proliferation and angiogenesis capabilities.52) Additionally, Akt modified MSCs show enhanced engraftment and fusion with host cardiomyocytes soon after transplantation.30) Further studies have demonstrated that combined effects of MSCs expressing Akt and Ang-1 can result in better survival, angiogenesis, and restoration of cardiac function on a global scale for a long period of time.52)53) Li et al.42) have also generated genetically modified MSCs by overexpressing pro-survival gene Bcl-2. As a result, they observed a significant reduction in apoptosis and an increase in expression level of VEGF. These effects were visible for a long time compared to normal MSC treatment. MSC-Bcl-2 transplanted animals also demonstrated reduction in the infarct zone and higher capillary density.42) Heme oxygenase-1 (HO-1) is another cytoprotective gene that ensures cell survival by inhibiting HMGB1 protein during conditions like inflammation and endotoxemia.54)55) Transfection of MSCs with HO-1 has resulted in capillary and arteriolar density increase in the peri-infarct zone.56) Another group has reported the survival of HO-1 modified MSCs is increased 5 folds as soon as 7 days after transplantation.56)57) To improve MSC migration, chemokine receptors such as CXCR or CCR have been employed.58) Huang et al.59) have demonstrated that overexpression of CCR1 in MSCs not only promotes cell migration, but also inhibits apoptosis and activates angiogenesis. Transplanted CCR1-MSCs in infarcted hearts can restore the cardiac function and induce an antifibrosis.59) Synergistically increasing cell adhesion of MSCs has also been attempted. For example, to enhance adhesion of MSCs in ischemic myocardium, integrin-linked kinase (ILK) has been overexpressed. Such ILK-MSCs can reduce infarcted size and fibrosis, increase microvessels, and assist cardiac recovery after injury.60) Advantages of this genetic modification approach include efficient induction of priming through stable expression of genes of interests and long remaining effects following priming application. Apart from enhanced therapeutic effects, safety is the most critical limitation for future clinical therapeutic use of genetically engineered MSCs since viral integration in host genome may increase tumorigenicity. Further studies that closely monitor the fate of transplanted gene-modified MSCs will be necessary to address such safety concern.
Oxygen is one of essential factors for the maintenance/survival of cells. Low or no supply of O2 can induce severe pathologies. Low oxygen availability decreases the viability and proliferation of MSCs. However, subsequent re-oxygenation can increase the cell survivability. This continuous process of hypoxia and re-oxygenation can stimulate the expression of various pro-survival genes, hence enabling MSCs to tolerate the harsh microenvironment in vivo. MSCs exposed to 1% oxygen condition show enhanced survivability and angiogenesis due to decreased apoptosis and enhanced expression of VEGF and FGF2 in a low-serum containing medium.44)61) MSCs cultured under a normoxia condition prior to hypoxia (0.5% O2) have demonstrated increased survival and better engraftment when they are implanted into infarcted heart tissues.62) These hypoxia preconditioned cells display increased expression of pro-angiogenesis, pro-survival, and pro-differentiation factors.62) Another study has demonstrated similar results, reporting that transplantation of preconditioned MSCs through hypoxia can enhance cardiac function via increased expression of pro-survival and pro-angiogenic factors like HIF1α, Bcl-xL, VEGF, and ANGPT1. On the other hand, expression levels of pro-apoptotic factors such as caspase-3 are significantly reduced after such transplantation.62)63)64)
Along with the expression of pro-survival and pro-angiogenic factors, it is also necessary for MSCs to precisely reach the site of infarction. Several previous studies have provided insight into the role of hypoxia priming in enhancing homing capabilities of transplanted MSCs.65)66) MSCs cultured under hypoxic (1–3% O2) conditions can trigger the expression of cMet, a primary receptor of hepatocyte growth factor (HGF) signaling pathway, and enhance the migration of cells towards the ischemic site.65) Similarly, another study has reported that the migration and homing capabilities of MSCs are improved after hypoxic treatment before transplantation into MI heart.66) It has been found that a low oxygen treatment can increase expression levels of potassium channel and FAK, resulting in better cell motility.66) In terms of proliferation, hypoxia priming with 2% oxygen can increase the expression of normal cellular prion protein (PrPc), subsequently activating PrPc-dependent JAK2 and STAT3 pathways and thus increasing the proliferative capacity of MSCs.67) Additionally, increased PrPc expression also inactivates the apoptosis pathway through the activation of catalase and superoxide dismutase enzymes.67) To be a safe therapeutic agent, MSCs must show genetic stability and low tumorigenic potential in vivo. MSCs cultured at low oxygen concentrations (3% O2) show prolonged life span.68) On the other hand, they show decreased DNA damage, less chromosomal aberration, less telomerase shortening, and less oxidative stress compared to cells cultured at normal O2 concentration, proving that hypoxia priming plays an important role in the genetic and chromosomal stability of MSCs.69) Several other studies have demonstrated that treating MSCs with lower oxygen concentration prior to transplantation can down-regulate the expression of specific genes involved in tumorigenesis, thus making MSCs a safe option for regenerative therapy.69)70)71)
There is no doubt that hypoxia-derived priming has many positive effects on MSCs in a number of ways, including improving their viability, proliferation, secretion potential for many angiogenic cytokines, and homing capability of MSCs. However, in order to make hypoxia-derived priming more effective, it is still necessary to optimize protocols such as finding the most effective oxygen concentration for reoxygenation and the optimal duration for hypoxia and reoxygenation.
Priming/preconditioning approaches using growth factors or cytokines can also improve angiogenic potential and survival of MSCs. Gorin et al.72) have demonstrated that fibroblast growth factor-2 (FGF-2) priming not only increases the secretion of VEGF and HGF, but also increases MSC-induced vascularization. Another study has reported that priming with SDF-1 can increase the survival and proliferation of MSCs in a significant manner.73) Treatment with SDF-1 can enhance the secretion of cytokines involved in cell survival by activating Akt and ERK signaling pathways.73) In addition, angiogenesis can be enhanced due to increased secretion of VEGF.73) Priming of MSCs with transforming growth factor (TGF)-α can increase angiogenesis as VEGF is over-expressed through p38 mitogen-activated protein kinase (MAPK)-dependent pathway. The recovery of myocardial function can also be enhanced post-ischemic injury.44)74)
To modify immunomodulatory effects, one of major advantages of MSCs, pro-inflammatory factors such as TNF-α, interleukin (IL)-1, interferon gamma (IFN-γ), and lipopolysaccharide (LPS) have been employed. These factors can promote MSCs to release anti-inflammatory signals, thus enhancing the immunosuppressive function of MSCs.75) Among these factors, IFN-γ has been intensively studied.76) Primed MSCs with IFN-γ and TNF-α not only can suppress T cell proliferation, but also can differentiate monocytes to IL-10-secreting M2 macrophages. Indoleamine 2,3-dioxygenase (IDO) upregulation followed by T cell and NK cell inhibition has been widely suggested as a mechanism of such effect.77) Primed MSCs can also increase the production of anti-immunomodulatory factors such as TGF-β and prostaglandin-E2 and promote the expression of co-stimulatory molecules as well as histocompatibility leukocyte antigen.78) Chinnadurai et al.79) have demonstrated that hMSCs induced by IFN-γ can inhibit T cell effector function. Specifically, Th1 cytokine production and T cells degranulation are inhibited.
In addition to IFN-γ, TNF-α and IL-1 have also been used to amplify immunomodulatory effects of MSCs. TNF-α is a pro-inflammatory cytokine that is usually combined with IFN-γ to stimulate MSCs.78) It can strengthen effects of IFN-γ on MSCs, improve the IDO activity, cytokine production, and T cell suppression of MSCs. Moreover, such combination could restore IDO1 mRNA level of MSCs after freeze-thawing.80) Primed MSC with IFN-γ and TNF-α can also affect innate immunity through H factor to inhibit complement activation.81) Unlike TNF-α, IL-1 can be applied alone to induce MSCs to treat stroke patients. Redondo-Castro et al.82) have reported that MSCs primed by IL-1α or IL-1β can release higher levels of granulocyte colony stimulating factor, IL-10, and other trophic factors than MSCs primed by IFN-γ or TNF-α. When tested with activated microglial cells, conditioned medium of MSC induced by IL-1 exhibits strong immunomodulatory effects, suggesting a promising therapy for stroke treatment.82) Other pro-inflammatory cytokines such as IL-17, IL-6, IL-23, TGF-β1, and their combinations have been intensively studied to improve therapeutic effects of MSCs.78) Priming MSCs with cytokines has generally been considered relatively easy and safe because cytokines do not alter genes in MSCs. However, in order to induce more efficient priming, it is necessary to find the optimal cytokine as a prerequisite. In addition, priming MSCs with cytokines is not cost-effective because it usually requires significant amounts of cytokines.
Several previous studies have reported that priming MSCs with distinct pharmacological agents can protect MSCs from ischemic damage by activating endogenous cellular machinery, thus further enhancing the therapeutic potential of MSCs.44) Pre-treatment of MSCs with an anti-ischemic drug trimetazidine (TMZ) can protect MSCs against oxidative stress-induced cell death.83) Pre-treatment of MSCs with TMZ increased expression levels of pro-survival factors such as HIF-1α, Akt, survivin, and Bcl-2.83) Overall improvement in cardiac function has been observed after transplantation of TMZ pre-treated MSCs.83) Another study has reported that vitamin E primed MSCs can survive hydrogen peroxide induced oxidative stress, thus increasing cell viability.84) In addition, priming MSCs with Atorvastatin (Statin) can improve their survival post-transplantation by activating endothelial nitric oxide synthase (eNOS).85) After transplantation of Atorvastatin treated MSCs, significant improvements in tissue repair and cardiac function have been observed.85) Priming MSCs with hormones can also enhance their survivability. MSCs treated by oxytocin (OT) have demonstrated higher survival from stresses induced by hypoxia and serum deprivation.86) Cell proliferation and migration are also improved as OT treatment up-regulates the expression of Akt and ERK1/2 proteins.86) Survival, proliferation, and migration of MSCs are improved in a hypoxia/serum deprivation in vitro model following treatment with an anesthetic drug sevoflurane.87) This treatment can reduce the apoptosis rate and mitochondrial membrane potential loss caused by hypoxia and serum deprivation by up-regulating HIF-1α, Akt, and VEGF.86) Another group has also found that LPS treatment can protect MSCs from apoptosis by inhibiting Cyc-c released due to hypoxia and serum deprivation.86)
Pretreatment with pharmacological agents can increase cell viability, as well as promote the differentiation and proliferation potential of MSCs. Pre-treatment of MSCs with angiotensin receptor blockers can improve the cardiomyogenic transdifferentiation efficiency (CTE) in a significant manner.88) Astragaloside IV (AS-IV) is a common Chinese drug for treating cardiovascular diseases. Priming with AS-IV has resulted in the differentiation and proliferation of MSCs by neutralizing TLR4 expression induced by high glucose conditions.89) Natural products such as apple extract can also be used for priming MSCs. Apple extract can promote the proliferation of MSCs via phosphorylation of ERK protein and enhanced expression of VEGF and IL-6.90) LL-37 is an anti-microbial agent found in wounds with a role in wound healing. Pre-treatment with LL-37 can enhance the proliferation and migration of MSCs by inducing over-expression of EGR1 and MAPK proteins.91) RU486 is another molecule that can promote the proliferation and differentiation of MSCs in a gender-dependent manner.92) The proliferation of MSCs from male donors is higher than that from female donors following treatment with RU486. The underlying mechanism of such proliferation and differentiation is the over-expression of FGF-2 and Sox-11 induced by RU486 pre-treatment. 92) Genistein is an active compound found in soy isoflavone. It is mostly involved in anti-oxidative and anti-inflammatory activities. Pre-treatment of MSCs with this compound can increase their proliferative capabilities in a dose and time-dependent manner by overexpressing peroxisome proliferator-activated receptor γ (PPARγ).93) Pre-treatment of MCSs with dimethyloxalylglycine can also facilitate the migration of MSCs into peripheral blood circulation, resulting in reduction of infarct size and the induction of myocardial repair following transplantation.94) Migration and homing abilities of MSCs are also increased after MSCs are treated with deferoxamine.95) The underlying mechanism that causes this improvement is the overexpression of specific chemokines and proteases such as CXCR4, CCR2, HIF1-α, and MMP-2/9.43)96)
Recent advances in biomechanical and tissue engineering have contributed to the development of systems that can effectively prime MSCs. Priming with silica nanoparticles can improve the proliferation of MSCs as these particles can induced phosphorylation of proteins in the ERK1/2 pathway.48) Priming with GFc7, a nano-chelator complex, can enhance proliferation and homing capabilities of MSCs.97) It also provides resistance against stress conditions and repression of spontaneous differentiation.97) Migration capabilities of MSCs are substantially improved after pre-treatment with topographic microstructures created on polystyrene culture vessel surfaces via activation of FAK and MAPK proteins.98) Priming MSCs with a composite hydrogel produced by combining silk fibroin and poly(ethylene glycol) diacrylate can improve their viability and differentiation.99) Neovascularization of MSCs has been found to be enhanced after priming with deferoxamine followed by their seeding on a collagen-glycosaminoglycan dermal substrate.100) Culturing of MSCs on a 3D culture which includes encapsulating cells in biomaterials like hydrogel, porous scaffolds, and scaffold-free spheroids can significantly enhance the adhesion pattern, migration, ECM expression, and secretion of paracrine factors of MSCs.101)102)103)104)
Photo-biomodulation is also one of methods used for priming MSCs. This method involves exposure of MSCs to low-intensity lasers. As a result, the proliferation of MSCs is increased due to increased mitochondrial biogenesis and up-regulation of growth factors such as HGF and PDGF.45) Pulsed electromagnetic fields (PEMF) can also bring key biological changes when MSCs are exposed to them, resulting in reduced apoptosis and enhanced survival in a time and dose-dependent manner. PEMF treatment can induce Akt/Ras signaling pathway, thus up-regulating the expression of pro-survival proteins such as Bcl-xL and Bad.46)
Priming with mechanical stretch can help MSCs survive better in a hostile environment, including nutrient deprivation via nuclear factor-κB pathway activation. An increase of angiogenesis is also evident from increased expression of VEGFA.71) Exposing MSCs to a high temperature for a certain period can also enhance the survival of MSCs post transplantation.105) Heat shock can result in the overexpression of heat shock proteins having an anti-apoptotic role.106)
Overall, priming MSCs with physical factors have several advantages, including a fast speed and a low cost. However, these physical factors have certain adverse effects on cells such as cell death and DNA damage. Moreover, they are limited to a small scale. Future studies are needed to optimize the protocol in terms of selecting optimal types of biomaterials and determining their cytotoxicity and biodegradability. Future studies should also focus on the underlying mechanism through which these materials can influence properties of MSCs.
Recently, Park et al.107) have reported an interesting strategy for priming MSCs in a 3-dimensional patch implanted in the heart in vivo. They claim that effects of the conventional priming strategy performed in vitro before cells are transplanted into the heart do not last long, typically only 2 or 3 days. To extend the duration of the priming effect, they have come up with an idea of ‘in vivo priming’, meaning that hMSCs are primed directly in the heart in situ. To achieve in vivo priming, they loaded bone marrow-derived hMSCs (BM-MSCs) with genetically engineered hepatocyte growth factor expressing MSCs (HGF-eMSCs) designed to continuously secret human HGF protein into a tailor-made 3D-printed patch. The rationale behind this was to prime BM-MSCs through HGF constantly released from HGF-eMSCs within the 3D cardiac patch implanted in the epicardium of the heart in vivo. HGF is known to be involved in multiple biological activities such as cell survival, blood vessel formation, and anti-fibrotic activities. It is essential for adult organ regeneration and wound healing.108) In terms of patch generation, the patch is bio-ink produced using pig heart-derived extracellular matrix to mimic the cardiac tissue-specific microenvironment. A rat MI model was used to examine therapeutic effects of in vivo priming. Echocardiography demonstrated that the therapeutic efficacy of the HGF-eMSCs/BM-MSCs combination group was higher than that of BM-MSCs only or the control group. Adverse cardiac remodeling was also reduced in the HGF-eMSCs/BM-MSCs group as compared to others. Such in vivo priming of BM-MSCs enhanced their survival. They remained viable for up to 8 weeks after implantation. In addition to survival, these primed BM-MSCs also enhanced vascular regeneration and protected the myocardium from ischemic insults. It was found that these primed hMSCs had a higher survival rate than unprimed ones in patches attached to failing hearts. These empowered hMSCs released greater amounts of paracrine factors beneficial for repairing damaged cardiac muscle tissues and regenerating vasculatures. These findings demonstrated the importance of hMSC based stem cell therapy in heart repair, emphasizing the inevitability of complex, systematic, and strategic application designs along with biomedical engineering technologies such as 3D printing rather than a single approach to achieve the goal of full recovery.
hMSC has been considered as one of the best cell types for cell-based cardiac regeneration therapy. A variety of previously mentioned strategies have been devised to improve the therapeutic potential of MSCs. However, MSCs can also increase the therapeutic potential of other cell types. For example, co-culture of MSCs with cardiomyocytes confers cytoprotective effects to these cardiomyocytes (CMs).109) Co-cultured CMs with MSCs have demonstrated enhanced phosphorylation of survival kinases including PKB/Akt and p-cAMP and reduce apoptosis against I/R injury.110) In addition to cardiomyocytes, bone marrow-derived endothelial progenitor cells co-cultured with hMSCs also show improved cell proliferation and enhanced angiogenic capacity through PDGF and Notch signaling pathways.111) Bone marrow-derived MSCs can also trigger the formation of cartilage from chondrocytes when they are co-cultured with chondrocytes. The formation of cartilage matrix is due to trophic factors secreted by BM-MSCs rather than the differentiation of MSCs into chondrocytes, independent of culture condition and origin.112) The viability and proliferation of erythroid progenitor cells are also significantly increased by co-culturing with MSCs.113) MSCs co-culture with neural stem cells (NSCs) can influence the proliferation of NSCs as confirmed by the up regulation of neuronal marker NSC.114) MSCs also play an important role in determining the cell fate of NSCs via the Notch signaling pathway.114)
Park et al.115) have recently reported another novel approach by loading MSCs into a 3D cardiac patch (hMSCs-PA) implanted epicardially in an MI induced rat heart and successfully primed intramyocardially injected CMs derived from human induced pluripotent stem cell (hiPSC-CMs). The rationale behind this dual stem cell approach is to repair both the myocardium and the vasculature as 2 major components in the heart that are severely damaged during MI condition. Authors sought to regenerate the myocardium using intramyocardially injected hiPSC-CMs. MSCs encapsulated in the implanted 3D patch are expected to regenerate vasculature attributed to paracrine factors released from MSCs. Of note, this dual stem cell approach has resulted in enhanced retention and engraftment of hiPSC-CMs. Previous studies involving the injection of cardiomyocytes have reported very low retention, engraftment, and survival of injected cells.116) The improved retention of hiPSC-CMs probably due to the fact that MSCs in the patch can secrete certain factors that enhance survival and maintenance, leading to engraftment of the injected CM. Interestingly, this dual approach also enhanced the maturity of injected hiPSC-CMs. Cytokines released from hMSCs-PA have emerged to be critical factors in the maturation of hiPSC-CMs. Indeed, practical application of hiPSC-CMs is limited by their immature phenotype. Many groups have come up with different mechanical, electrical, biochemical, and physical methods for the maturation of hiPSC-CMs. However, these methods were limited by factors such as scalability, damage to CMs, and clinical compatibility.2) Interestingly, with the dual stem cell approach, hiPSC-CMs injected along with hMSCs-PA (CM+PA) exhibited a rod-shaped morphology of mature CMs as compared to a globular morphology of immature hiPSCs-CMs injected alone. This fact was further confirmed that conditioned media taken from hMSC cultures improved the maturity of neonatal rat CM in vitro.115) The maturity of the CM+PA group was also evident from gap junction formation between injected CMs and the host myocardium. In addition to structural maturation, functional maturation was also visible from synchronized action potential, indicating great consensus between these 2 CMs.
In addition to priming of hiPSC-CMs, hMSCs-PA played a vital role in vascular regeneration in MI hearts as evident from overexpression of many angiogenesis-related genes. Based on histological evidence, functional capillary density in the infarct and border zone was significantly higher in the CM+PA group than in the hiPSC-CMs only group.115) TUNEL assay verified the fact that the mentioned improvement in angiogenesis was only attributed to hMSCs-PA, as most MSCs were viable and well within the patch.115) Along with survival and retention of injected CMs, the CM+PA approach also conferred cytoprotective effects on host cardiomyocytes due to the release of pro-survival factors from hMSCs-PA. In vitro experiments demonstrated that treatment of CMs with 10% hMSC-CM conditioned medium protected CMs against H2O2 induced ischemic injury.115) Furthermore, this approach also enhanced the migration of CMs. Collectively, authors confirmed that paracrine factors released from hMSC-PA comprised major contributors to multiple effects necessary for damaged heart recoveries, such as anti-inflammatory, pro-angiogenesis, anti-fibrosis, and CM maturity.
hMSCs have shown great promises in cell-based cardiac regeneration therapy due to their many advantages over other cell types. However, contrary to animal models, hMSCs demonstrated a modest degree of success in human clinical trials. The knowledge and lessons gained from clinical trials have paved the way for many innovations to enhance the potential of hMSCs as sources for cell-based therapy. Concerted efforts have been made in recent years to develop more sophisticated and efficient strategies to promote the therapeutic potential of hMSCs. Current approaches to enhance the therapeutic efficacy of hMSCs include genetic modifications, biological, pharmacological, chemical, physical strategies. Recent trends that combine several existing strategies have achieved incremental improvements for generating more therapeutically potent hMSCs. All these methods can enhance proliferation, differentiation, survival, engraftment, and therapeutic potential of MSCs. Promising results from pioneering in vivo priming approach have suggested a new direction for MSC-based cell therapy. Additionally, MSCs in a co-culture system can prime other cell types, demonstrating their versatile nature.
In summary, technological advances on hMSCs have opened a new chapter for realistic application of hMSCs for cardiac regeneration therapy. Although initial success has been achieved, several limitations such as scalability, and clinical compatibility remain to be answered. Extensive future studies are needed to develop more innovative approaches that could efficiently generate therapeutically effective hMSCs.
Notes
References
1. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015; 6:187–214. PMID: 26756631.


2. Jiang Y, Park P, Hong SM, Ban K. Maturation of cardiomyocytes derived from human pluripotent stem cells: current strategies and limitations. Mol Cells. 2018; 41:613–621. PMID: 29890820.


3. Sunagawa G, Horvath DJ, Karimov JH, Moazami N, Fukamachi K. Future prospects for the total artificial heart. Expert Rev Med Devices. 2016; 13:191–201. PMID: 26732059.


4. Miyagawa S, Fukushima S, Imanishi Y, et al. Building a new treatment for heart failure-transplantation of induced pluripotent stem cell-derived cells into the heart. Curr Gene Ther. 2016; 16:5–13. PMID: 26785736.


5. Anastasiadis K, Antonitsis P, Argiriadou H, et al. Hybrid approach of ventricular assist device and autologous bone marrow stem cells implantation in end-stage ischemic heart failure enhances myocardial reperfusion. J Transl Med. 2011; 9:12. PMID: 21247486.



7. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976; 4:267–274. PMID: 976387.

8. Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003; 412:196–212.

9. Fong CY, Chak LL, Biswas A, et al. Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev Rep. 2011; 7:1–16. PMID: 20602182.


10. Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003; 35:1151–1156. PMID: 12757751.


11. Shen D, Cheng K, Marbán E. Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction. J Cell Mol Med. 2012; 16:2112–2116. PMID: 22225626.



12. Menasché P, Vanneaux V. Stem cells for the treatment of heart failure. Curr Res Transl Med. 2016; 64:97–106. PMID: 27316393.

13. Ghiroldi A, Piccoli M, Cirillo F, et al. Cell-based therapies for cardiac regeneration: a comprehensive review of past and ongoing strategies. Int J Mol Sci. 2018; 19:3194.


14. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011; 109:923–940. PMID: 21960725.


15. Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007; 104:1643–1648. PMID: 17251350.



16. Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004; 95:9–20. PMID: 15242981.


17. Kim J, Shapiro L, Flynn A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacol Ther. 2015; 151:8–15. PMID: 25709098.


18. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749. PMID: 17656645.


19. Wang X, Zhen L, Miao H, et al. Concomitant retrograde coronary venous infusion of basic fibroblast growth factor enhances engraftment and differentiation of bone marrow mesenchymal stem cells for cardiac repair after myocardial infarction. Theranostics. 2015; 5:995–1006. PMID: 26155315.



20. Li X, Zhao H, Qi C, Zeng Y, Xu F, Du Y. Direct intercellular communications dominate the interaction between adipose-derived MSCs and myofibroblasts against cardiac fibrosis. Protein Cell. 2015; 6:735–745. PMID: 26271509.



21. Miteva K, Pappritz K, El-Shafeey M, et al. Mesenchymal stromal cells modulate monocytes trafficking in coxsackievirus B3-induced myocarditis. Stem Cells Transl Med. 2017; 6:1249–1261. PMID: 28186704.



22. Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015; 182:349–360. PMID: 25590961.


23. Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005; 11:367–368. PMID: 15812508.

24. Davani S, Marandin A, Mersin N, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003; 108(Suppl 1):II253–II258. PMID: 12970242.

25. Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020; 11:349. PMID: 32393744.



26. Pandey AC, Lancaster JJ, Harris DT, Goldman S, Juneman E. Cellular therapeutics for heart failure: focus on mesenchymal stem cells. Stem Cells Int. 2017; 2017:9640108. PMID: 29391871.

27. Liao S, Zhang Y, Ting S, et al. Potent immunomodulation and angiogenic effects of mesenchymal stem cells versus cardiomyocytes derived from pluripotent stem cells for treatment of heart failure. Stem Cell Res Ther. 2019; 10:78. PMID: 30845990.



28. Korf-Klingebiel M, Kempf T, Sauer T, et al. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008; 29:2851–2858. PMID: 18953051.


29. Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009; 106:14022–14027. PMID: 19666564.



30. Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006; 14:840–850. PMID: 16965940.


31. Zhao JJ, Liu XC, Kong F, et al. Bone marrow mesenchymal stem cells improve myocardial function in a swine model of acute myocardial infarction. Mol Med Rep. 2014; 10:1448–1454. PMID: 25060678.


32. Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004; 94:92–95. PMID: 15219514.


33. Lee JW, Lee SH, Youn YJ, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014; 29:23–31. PMID: 24431901.


34. Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015; 36:1744–1753. PMID: 25926562.


35. Xiao W, Guo S, Gao C, et al. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017; 58:238–244. PMID: 28190794.


36. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364:141–148. PMID: 15246726.

37. Gyöngyösi M, Wojakowski W, Lemarchand P, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015; 116:1346–1360. PMID: 25700037.



38. Yu H, Lu K, Zhu J, Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017; 121:135–154. PMID: 28164211.


39. Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014; 114:1302–1310. PMID: 24565698.


40. Guijarro D, Lebrin M, Lairez O, et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the MESAMI 1 pilot trial. Int J Cardiol. 2016; 209:258–265. PMID: 26901787.


41. Florea V, Rieger AC, DiFede DL, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study). Circ Res. 2017; 121:1279–1290. PMID: 28923793.


42. Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007; 25:2118–2127. PMID: 17478584.


43. Najafi R, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013; 13:959–972. PMID: 23536977.

44. Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo
. J Cell Mol Med. 2018; 22:1428–1442. PMID: 29392844.


45. Yin K, Zhu R, Wang S, Zhao RC. Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev. 2017; 26:762–775. PMID: 28178868.


46. Urnukhsaikhan E, Cho H, Mishig-Ochir T, Seo YK, Park JK. Pulsed electromagnetic fields promote survival and neuronal differentiation of human BM-MSCs. Life Sci. 2016; 151:130–138. PMID: 26898125.


47. Wang S, Zhang C, Niyazi S, et al. A novel cytoprotective peptide protects mesenchymal stem cells against mitochondrial dysfunction and apoptosis induced by starvation via Nrf2/Sirt3/FoxO3a pathway. J Transl Med. 2017; 15:33. PMID: 28202079.

48. Kim KJ, Joe YA, Kim MK, et al. Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through ERK1/2 activation. Int J Nanomedicine. 2015; 10:2261–2272. PMID: 25848249.



49. Fierro FA, O'Neal AJ, Beegle JR, et al. Hypoxic pre-conditioning increases the infiltration of endothelial cells into scaffolds for dermal regeneration pre-seeded with mesenchymal stem cells. Front Cell Dev Biol. 2015; 3:68. PMID: 26579521.



50. Matsui T, Tao J, del Monte F, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001; 104:330–335. PMID: 11457753.

51. Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9:1195–1201. PMID: 12910262.


52. Jiang S, Haider HK, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006; 99:776–784. PMID: 16960098.


53. Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008; 77:525–533. PMID: 18032392.


54. Wang J, Yang H, Hu X, et al. Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo
. J Surg Res. 2013; 183:509–516. PMID: 23531454.

55. Takamiya R, Hung CC, Hall SR, et al. High-mobility group box 1 contributes to lethality of endotoxemia in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol. 2009; 41:129–135. PMID: 19097991.


56. Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005; 46:1339–1350. PMID: 16198853.


57. Jiang YB, Zhang XL, Tang YL, et al. Effects of heme oxygenase-1 gene modulated mesenchymal stem cells on vasculogenesis in ischemic swine hearts. Chin Med J (Engl). 2011; 124:401–407. PMID: 21362341.

58. De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016; 8:73–87. PMID: 27022438.


59. Huang J, Zhang Z, Guo J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010; 106:1753–1762. PMID: 20378860.



60. Song SW, Chang W, Song BW, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009; 27:1358–1365. PMID: 19489098.


61. Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013; 37:551–560. PMID: 23505143.


62. Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008; 135:799–808. PMID: 18374759.


63. Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010; 299:C1562–70. PMID: 20861473.

64. Hu X, Xu Y, Zhong Z, et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ Res. 2016; 118:970–983. PMID: 26838793.


65. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008; 26:2173–2182. PMID: 18511601.



66. Hu X, Wei L, Taylor TM, et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011; 301:C362–72. PMID: 21562308.

67. Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016; 7:e2395. PMID: 27711081.

68. Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007; 6:745–757. PMID: 17925003.


69. Estrada JC, Albo C, Benguría A, et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012; 19:743–755. PMID: 22139129.


70. Singec I, Snyder EY. Inflammation as a matchmaker: revisiting cell fusion. Nat Cell Biol. 2008; 10:503–505. PMID: 18454127.


71. Zhu Z, Gan X, Fan H, Yu H. Mechanical stretch endows mesenchymal stem cells stronger angiogenic and anti-apoptotic capacities via NFκB activation. Biochem Biophys Res Commun. 2015; 468:601–605. PMID: 26545780.

72. Gorin C, Rochefort GY, Bascetin R, et al. Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Transl Med. 2016; 5:392–404. PMID: 26798059.



73. Liu X, Duan B, Cheng Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011; 2:845–854. PMID: 22058039.



74. Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010; 33:24–30. PMID: 19996917.


75. Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018; 38:1276–1292. PMID: 29768965.



76. Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006; 24:386–398. PMID: 16123384.

77. François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012; 20:187–195. PMID: 21934657.


78. Noronha NC, Mizukami A, Caliári-Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019; 10:131. PMID: 31046833.



79. Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol. 2014; 192:1491–1501. PMID: 24403533.


80. Rovira Gonzalez YI, Lynch PJ, Thompson EE, Stultz BG, Hursh DA.
In vitro cytokine licensing induces persistent permissive chromatin at the indoleamine 2,3-dioxygenase promoter. Cytotherapy. 2016; 18:1114–1128. PMID: 27421739.


81. Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010; 19:1803–1809. PMID: 20163251.



82. Redondo-Castro E, Cunningham C, Miller J, et al. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro
. Stem Cell Res Ther. 2017; 8:79. PMID: 28412968.



83. Wisel S, Khan M, Kuppusamy ML, et al. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009; 329:543–550. PMID: 19218529.

84. Bhatti FU, Mehmood A, Latief N, et al. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2017; 25:321–331. PMID: 27693502.

85. Song L, Yang YJ, Dong QT, et al. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One. 2013; 8:e65702. PMID: 23741509.

86. Noiseux N, Borie M, Desnoyers A, et al. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012; 153:5361–5372. PMID: 23024264.


87. Sun X, Fang B, Zhao X, Zhang G, Ma H. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS One. 2014; 9:e90667. PMID: 24599264.

88. Numasawa Y, Kimura T, Miyoshi S, et al. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011; 29:1405–1414. PMID: 21755575.


89. Li M, Yu L, She T, et al. Astragaloside IV attenuates Toll-like receptor 4 expression via NF-κB pathway under high glucose condition in mesenchymal stem cells. Eur J Pharmacol. 2012; 696:203–209. PMID: 23041150.

90. Lee J, Shin MS, Kim MO, et al. Apple ethanol extract promotes proliferation of human adult stem cells, which involves the regenerative potential of stem cells. Nutr Res. 2016; 36:925–936. PMID: 27632912.


91. Yang Y, Choi H, Seon M, Cho D, Bang SI. LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway. Stem Cell Res Ther. 2016; 7:58. PMID: 27095351.



92. Yu Y, Wei N, Stanford C, Schmidt T, Hong L.
In vitro effects of RU486 on proliferation and differentiation capabilities of human bone marrow mesenchymal stromal cells. Steroids. 2012; 77:132–137. PMID: 22093480.

93. Zhang LY, Xue HG, Chen JY, Chai W, Ni M. Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARγ. Exp Ther Med. 2016; 11:1853–1858. PMID: 27168816.



94. Liu XB, Wang JA, Ji XY, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther. 2014; 5:111. PMID: 25257482.


95. Peyvandi AA, Abbaszadeh HA, Roozbahany NA, et al. Deferoxamine promotes mesenchymal stem cell homing in noise-induced injured cochlea through PI3K/AKT pathway. Cell Prolif. 2018; 51:e12434. PMID: 29341316.

96. Fujisawa K, Takami T, Okada S, et al. Analysis of metabolomic changes in mesenchymal stem cells on treatment with desferrioxamine as a hypoxia mimetic compared with hypoxic conditions. Stem Cells. 2018; 36:1226–1236. PMID: 29577517.

97. Hafizi M, Hajarizadeh A, Atashi A, et al. Nanochelating based nanocomplex, GFc7, improves quality and quantity of human mesenchymal stem cells during in vitro expansion. Stem Cell Res Ther. 2015; 6:226. PMID: 26597909.



98. Li Z, Xu X, Wang W, et al. Modulation of the mesenchymal stem cell migration capacity via preconditioning with topographic microstructure. Clin Hemorheol Microcirc. 2017; 67:267–278. PMID: 28869459.


99. Ciocci M, Cacciotti I, Seliktar D, Melino S. Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems. Int J Biol Macromol. 2018; 108:960–971. PMID: 29113887.


100. Wahl EA, Schenck TL, Machens HG, Balmayor ER. VEGF released by deferoxamine preconditioned mesenchymal stem cells seeded on collagen-GAG substrates enhances neovascularization. Sci Rep. 2016; 6:36879. PMID: 27830734.



101. Sart S, Agathos SN, Li Y, Ma T. Regulation of mesenchymal stem cell 3D microenvironment: from macro to microfluidic bioreactors. Biotechnol J. 2016; 11:43–57. PMID: 26696441.


102. Maia FR, Fonseca KB, Rodrigues G, Granja PL, Barrias CC. Matrix-driven formation of mesenchymal stem cell-extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 2014; 10:3197–3208. PMID: 24607421.


103. Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog. 2004; 20:905–912. PMID: 15176898.


104. Tsai AC, Liu Y, Ma T. Expansion of human mesenchymal stem cells in fibrous bed bioreactor. Biochem Eng J. 2016; 108:51–57.

105. Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther. 2019; 10:348. PMID: 31771642.



106. Moloney TC, Hoban DB, Barry FP, Howard L, Dowd E. Kinetics of thermally induced heat shock protein 27 and 70 expression by bone marrow-derived mesenchymal stem cells. Protein Sci. 2012; 21:904–909. PMID: 22505291.



107. Park BW, Jung SH, Das S, et al.
In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020; 6:eaay6994. PMID: 32284967.
108. Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad, Ser B, Phys Biol Sci. 2010; 86:588–610.

109. Xu RX, Chen X, Chen JH, Han Y, Han BM. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009; 36:176–180. PMID: 18785984.

110. Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008; 51:933–943. PMID: 18308163.


111. Liang T, Zhu L, Gao W, et al. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio. 2017; 7:1722–1736.



112. Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012; 18:1542–1551. PMID: 22429306.

113. Lazar-Karsten P, Dorn I, Meyer G, Lindner U, Driller B, Schlenke P. The influence of extracellular matrix proteins and mesenchymal stem cells on erythropoietic cell maturation. Vox Sang. 2011; 101:65–76. PMID: 21175667.


114. Wang Y, Tu W, Lou Y, et al. Mesenchymal stem cells regulate the proliferation and differentiation of neural stem cells through Notch signaling. Cell Biol Int. 2009; 33:1173–1179. PMID: 19706332.


115. Park SJ, Kim RY, Park BW, et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019; 10:3123. PMID: 31311935.



116. Müller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002; 34:107–116. PMID: 11851351.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download